Electrochemically-Formed Disordered Rock Salt ω-LixV9Mo6O40 as a Fast-Charging Li-Ion Electrode Material
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Electrochemically-formed disordered rock salt compounds are an emerging class of Li-ion electrode materials for fast-charging energy storage. However, the specific factors that govern the formation process and the resulting charge storage performance are not well understood. Here, we characterize the transformation mechanism and charge storage properties of an electrochemically-formed disordered rock salt from V9Mo6O40 (VMO). The crystal structure of VMO has similar motifs to that of α-V2O5, a well-studied analogue, but VMO has less mechanical flexibility due to additional corner-sharing octahedra in its structure. As a result, VMO undergoes a single-step transformation pathway, which we characterize through operando X-ray diffraction, and forms an unusual highly distorted lamellar microstructure, as we show with high-resolution transmission electron microscopy. The resulting LixVMO material shows fast charging and other electrochemical characteristics and performance typical of many nanomaterials, even though the material is composed of relatively large particles.
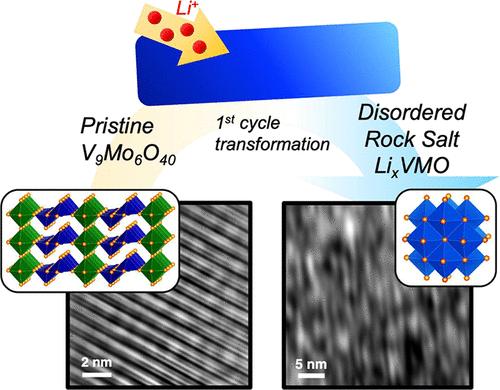
电化学形成的无序岩盐ω-LixV9Mo6O40作为快速充电锂离子电极材料
电化学形成的无序岩盐化合物是一类新兴的用于快速充电储能的锂离子电极材料。然而,控制形成过程和由此产生的电荷存储性能的具体因素尚不清楚。本文研究了一种由V9Mo6O40 (VMO)电化学生成的无序岩盐的转变机理和电荷存储特性。VMO的晶体结构与α-V2O5具有相似的基序,但由于其结构中附加了共享角的八面体,VMO的机械柔韧性较差。结果,我们通过operando x射线衍射表征了VMO的单步转变途径,并形成了不寻常的高度扭曲的层状微观结构,正如我们通过高分辨率透射电子显微镜所显示的那样。所得的LixVMO材料显示出快速充电和其他电化学特性以及许多纳米材料的典型性能,尽管该材料由相对较大的颗粒组成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


