Quantification of activated carbon functional groups and active surface area by TPD-MS and their impact on supercapacitor performance
IF 20.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
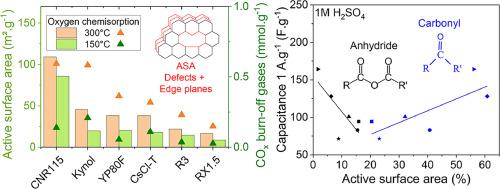
Carbon oxygenated functional groups and active sites play an important role in the interactions with the electrolytes in aqueous supercapacitors. For the first time, correlations between each type of O-surface groups and electrochemical performance are established by means of thermodesorption coupled with mass spectrometry (TPD-MS). A set of five activated carbons and one soft-salt templated carbon, were studied in three different pH electrolytes, 1 M H2SO4, 1 M KOH and 1 M Na2SO4. Linear correlations between surface groups and capacitance were found: acidic groups such as carboxylic acid and phenol-ether groups improve capacitance, whereas carbonyl-quinone groups are detrimental. Moreover, active surface area (ASA) is for the first time measured for activated carbons thanks to a new protocol, which minimises material burn-off during oxygen chemisorption. In addition, a new approach consisting in the quantification of the ASA is proposed. It has been highlighted that certain active sites are linearly correlated to an improvement of capacitance. Although the oxygen surface groups and ASA improve the capacitance via pseudo-capacitance phenomena, the capacitive mechanisms, governed by the porosity of the activated carbons, are shown to be predominant. Among all materials, the soft-salt templated carbon gives the best electrochemical performance. Indeed, it combines a large quantity of carboxylic acid and phenol-ether surface groups as well as appropriate ASA. Moreover, it has a high specific surface area (2556 m²·g-1) and optimal pore size (0.89 nm). All these characteristics, provide a high capacitance, a high rate capability and a high capacitance retention after 10,000 cycles.

TPD-MS定量活性炭官能团和活性表面积及其对超级电容器性能的影响
在水性超级电容器中,碳氧官能团和活性位点在与电解质的相互作用中起着重要作用。首次利用热脱附-质谱(TPD-MS)方法建立了各类型o -表面基团与电化学性能之间的相关性。研究了5种活性炭和1种软盐模板炭在不同pH的电解液(1M H2SO4、1M KOH和1M Na2SO4)中的性能。表面基团与电容之间存在线性关系:酸性基团如羧酸和酚醚基团改善电容,而羰基醌基团则有害。此外,由于一种新的方案,首次测量了活性炭的活性表面积(ASA),该方案最大限度地减少了氧化学吸附过程中材料的燃烧。此外,本文还提出了一种新的ASA量化方法。已经强调,某些活性位点与电容的改善呈线性相关。虽然氧表面基团和ASA通过赝电容现象改善了电容,但电容机制主要受活性炭孔隙率的控制。在所有材料中,软盐模板炭的电化学性能最好。事实上,它结合了大量的羧酸和酚醚表面基团以及适当的ASA。此外,它还具有较高的比表面积(2556 m²·g-1)和最佳孔径(0.88 nm)。所有这些特点,提供了高电容,高速率能力和高电容保持10000次循环后。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


