Amygdala intercalated cells form an evolutionarily conserved system orchestrating brain networks
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
The amygdala attributes valence and emotional salience to environmental stimuli and regulates how these stimuli affect behavior. Within the amygdala, a distinct class of evolutionarily conserved neurons form the intercalated cell (ITC) clusters, mainly located around the boundaries of the lateral and basal nuclei. Here, we review the anatomical, physiological and molecular characteristics of ITCs, and detail the organization of ITC clusters and their connectivity with one another and other brain regions. We describe how ITCs undergo experience-dependent plasticity and discuss emerging evidence demonstrating how ITCs are innervated and functionally regulated by neuromodulatory systems. We summarize recent findings showing that experience alters the balance of activity between different ITC clusters, thereby determining prevailing behavioral output. Finally, we propose a model in which ITCs form a key system for integrating divergent inputs and orchestrating brain-wide circuits to generate behavioral states attuned to current environmental circumstances and internal needs. This comprehensive review of amygdala intercalated cells (ITCs) proposes a revised model of ITCs as a highly coordinated system that integrates diverse inputs to orchestrate brain-wide networks and shape behavioral states.

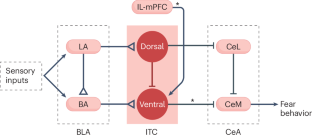
杏仁核嵌入细胞形成了一个进化上保守的协调大脑网络的系统
杏仁核会对环境刺激赋予价值和情绪显著性,并调节这些刺激对行为的影响。在杏仁核内,一类与众不同的进化保守神经元形成了夹层细胞(ITC)群,主要位于侧核和基底核的边界周围。在此,我们回顾了ITC的解剖、生理和分子特征,并详细介绍了ITC簇的组织结构及其与其他脑区的连接。我们描述了ITC如何经历依赖经验的可塑性,并讨论了证明ITC如何受神经调节系统支配和功能调节的新证据。我们总结了最近的研究结果,这些结果表明经验会改变不同 ITC 簇之间的活动平衡,从而决定主要的行为输出。最后,我们提出了一个模型,在该模型中,ITC 是整合不同输入和协调全脑回路的关键系统,可产生与当前环境和内部需求相适应的行为状态。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


