Metabolic rearrangement enables adaptation of microbial growth rate to temperature shifts
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Temperature is a key determinant of microbial behaviour and survival in the environment and within hosts. At intermediate temperatures, growth rate varies according to the Arrhenius law of thermodynamics, which describes the effect of temperature on the rate of a chemical reaction. However, the mechanistic basis for this behaviour remains unclear. Here we use single-cell microscopy to show that Escherichia coli exhibits a gradual response to temperature upshifts with a timescale of ~1.5 doublings at the higher temperature. The response was largely independent of initial or final temperature and nutrient source. Proteomic and genomic approaches demonstrated that adaptation to temperature is independent of transcriptional, translational or membrane fluidity changes. Instead, an autocatalytic enzyme network model incorporating temperature-sensitive Michaelis–Menten kinetics recapitulates all temperature-shift dynamics through metabolome rearrangement, resulting in a transient temperature memory. The model successfully predicts alterations in the temperature response across nutrient conditions, diverse E. coli strains from hosts with different body temperatures, soil-dwelling Bacillus subtilis and fission yeast. In sum, our model provides a mechanistic framework for Arrhenius-dependent growth. Growth rate dynamics after temperature shifts can be explained by metabolic rearrangement due to temperature-sensitive enzyme activities in bacteria and yeast.
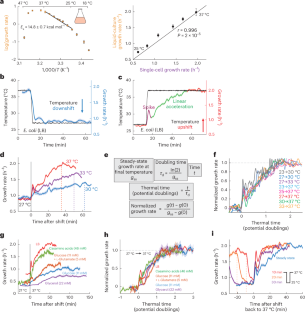

代谢重排使微生物生长速度适应温度变化
温度是决定微生物在环境中和宿主体内的行为和生存的关键因素。在中间温度下,生长速度根据热力学的阿伦尼乌斯定律而变化,该定律描述了温度对化学反应速度的影响。然而,这种行为的机理基础仍不清楚。在这里,我们利用单细胞显微镜显示,大肠埃希氏菌对温度升高表现出渐进的反应,在较高温度下的时间尺度约为 1.5 倍。这种反应在很大程度上与初始或最终温度以及营养源无关。蛋白质组和基因组方法表明,对温度的适应与转录、翻译或膜流动性变化无关。相反,一个包含对温度敏感的迈克尔-门顿动力学的自催化酶网络模型通过代谢组的重新排列再现了所有温度变化动态,从而产生了短暂的温度记忆。该模型成功预测了不同营养条件下的温度响应变化、来自不同体温宿主的不同大肠杆菌菌株、土壤中的枯草芽孢杆菌和裂殖酵母。总之,我们的模型为阿伦尼乌斯依赖性生长提供了一个机理框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


