DPP4 promotes an immunoenhancing tumor microenvironment through exhausted CD8+ T cells with activating IL13-IL13RA2 axis in papillary thyroid cancer
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
Abstract
Background
Papillary thyroid cancer (PTC) is among the most prevalent forms of endocrine malignancy with a rapid rise in incidence rates worldwide; however, the composition and characteristics of its immune microenvironment is poorly understand. Here, this work investigated the precise function of Dipeptidyl peptidase 4 (DPP4) in tumor-infiltrated T cells within PTC by investigating its role in cytokine-mediated signaling pathways.
Methods
TCGA and GEO data as well as human PTC specimens confirmed the expression of DPP4 in PTC. The CIBERSORT and TIMER tool were used to analyze the distribution of tumor-infiltrating immune cells in PTC. CD8+ T cells from PTC patient’s peripheral blood were cultured and used in a three-dimensional model for direct co-culture with PTC tumors to investigate DPP4 function.
Results
Bioinformatic analyses has uncovered a significant upregulation of DPP4, which enhances the survival and migration of PTC cells in vitro. DPP4 upregulation significantly correlated with advanced grades, stages, and poor progression-free survival. DPP4 influences immune function and the exhaustion of CD8+ T cells through the IL13-IL13RA2 axis. The inhibition of DPP4 reduces CD8+ T cell exhaustion and IL13 secretion, while also blocking the IL13-IL13RA2 axis, thereby promoting the mesenchymal-to-epithelial transition of PTC cells.
Conclusion
Blocking DPP4 leads to the conversion of exhausted CD8+ T cells with decreased IL13 level, resulting in downregulation of IL13RA2 to promote mesenchymal-to-epithelial transition of PTC cells. This highlights DPP4 as a potential therapeutic target, particularly between CD8+ T cells and PTC cells via IL13-IL13RA2 axis, and represents a novel avenue for combined immunotherapy in PTC.
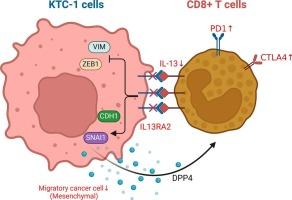
在乳头状甲状腺癌中,DPP4通过激活IL13-IL13RA2轴,通过耗尽的CD8+ T细胞促进免疫增强的肿瘤微环境。
背景:甲状腺乳头状癌(PTC)是最常见的内分泌恶性肿瘤之一,在世界范围内发病率迅速上升;然而,对其免疫微环境的组成和特点了解甚少。本研究通过研究二肽基肽酶4 (DPP4)在细胞因子介导的信号通路中的作用,研究了DPP4在PTC内肿瘤浸润T细胞中的确切功能。方法:TCGA和GEO数据以及人PTC标本证实DPP4在PTC中的表达。采用CIBERSORT和TIMER工具分析肿瘤浸润性免疫细胞在PTC中的分布。培养PTC患者外周血CD8+ T细胞,建立三维模型,与PTC肿瘤直接共培养,研究DPP4的功能。结果:生物信息学分析发现DPP4显著上调,提高了PTC细胞的体外存活和迁移能力。DPP4上调与晚期、分期和不良无进展生存期显著相关。DPP4通过IL13-IL13RA2轴影响免疫功能和CD8+ T细胞的衰竭。抑制DPP4可减少CD8+ T细胞耗竭和IL13分泌,同时阻断IL13- il13ra2轴,从而促进PTC细胞间质向上皮的转变。结论:阻断DPP4可导致耗竭的CD8+ T细胞转化,IL13水平降低,从而下调IL13RA2,促进PTC细胞间质向上皮转化。这突出了DPP4作为一个潜在的治疗靶点,特别是在CD8+ T细胞和PTC细胞之间通过IL13-IL13RA2轴,代表了PTC联合免疫治疗的新途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


