Organocatalytic Hat Trick: 1,5,7-Triazabicyclo[4.4.0]dec-5-ene (TBD)-Catalyzed Synthesis of Cyclic Imides via an Amidation–Cyclization–Elimination Cascade
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
1,5,7-Triazabicyclo[4.4.0]dec-5-ene (TBD) was used for the synthesis of cyclic imides via an amidation–cyclization–elimination cascade. This organocatalytic transformation features both the traditional reactivity of TBD and its unprecedented C–C bond cleavage capability, allowing rapid and efficient access to cyclic imides. This method is compatible with the late-stage functionalization of complex molecules and the synthesis of bioactive molecules. Both experimental and computational approaches were employed to gain a better understanding of the reaction mechanism.
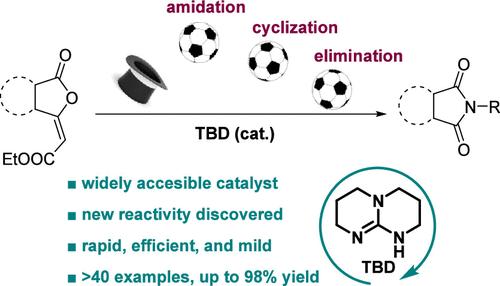
有机催化的帽子戏法:1,5,7-三氮杂环[4.4.0]十二-5-烯(TBD)通过酰胺化-环化-消除级联催化合成环酰亚胺
1,5,7-三氮杂比环[4.4.0]十二-5-烯(TBD)通过酰胺化-环化-消除级联反应合成了环亚胺。这种有机催化转化既具有TBD的传统反应性,又具有前所未有的C-C键裂解能力,可以快速有效地获得环亚胺。该方法适用于复杂分子的后期功能化和生物活性分子的合成。为了更好地了解反应机理,采用了实验和计算两种方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


