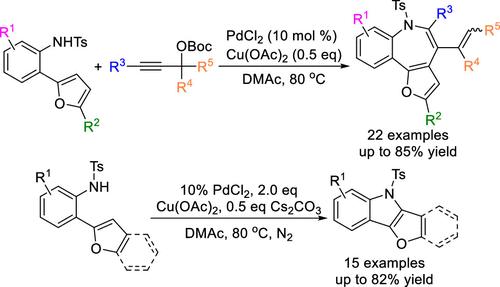
Controllable Synthesis of Benzo[b]furo[2,3-d]azepines or Furo[3,2-b]indoles via Intermolecular Oxidative Annulation of 2-(Furan-2-yl)anilines and Propargyl Carbonates versus Intramolecular C–H Amination Reactions
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Two novel Pd-catalyzed protocols for the controllable synthesis of benzo[b]furo[2,3-d]azepines and furo[3,2-b]indoles have been developed by intermolecular oxidative annulation of 2-(furan-2-yl)anilines and propargyl carbonates versus intramolecular C–H amination reactions. These two protocols feature great scalability, functional group tolerance, and relatively mild reaction conditions. Notably, the robust methodologies could also provide valuable opportunities for assembling azepine-fused benzothiophene, indole-fused benzothiophene, and indole-fused benzimidazole, which may have potential applications in the synthesis of related pharmaceuticals or polymeric materials.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


