Unveiling the Antithermal Quenching Behavior in 0D Inorganic Metal Halide Cs2InCl5(H2O) Mediated by Upconversion Emission
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Inorganic metal halides (IMHs) often suffer from severe fluorescence thermal quenching, limiting their application at elevated temperatures. Therefore, the exploration of IMHs exhibiting antithermal quenching (ATQ) behavior is of great importance. In this study, we developed a green synthetic route using a solvent evaporation method to successfully synthesize the 0D IMHs Cs2InCl5(H2O). By precise control over the doping ratios of Sb3+, Yb3+, and Er3+, unique dual-mode emission properties are obtained. As the temperature increases, the compound exhibited downconversion and upconversion luminescence, with relative sensitivity SR-max values of 7.11% K–1 and 1.21% K–1, respectively. Particularly anomalous is the compound’s manifestation of an unconventional ATQ behavior during the upconversion process. In situ structural analysis confirmed that under high-temperature conditions, the 0D Cs2InCl5(H2O) metal halide undergoes structural evolution, transitioning through a Cs3In2Cl9 phase, which is responsible for the ATQ. This study provides experimental evidence for the abnormal ATQ of 0D metal halides, offering new inspiration for the multifunctionalization of 0D metal halides in high-temperature temperature sensing and dual-mode luminescence.
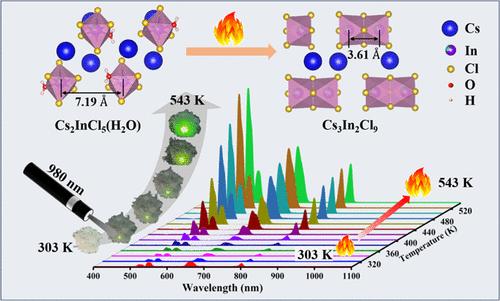
上转换发射介导0D无机金属卤化物Cs2InCl5(H2O)的抗热猝灭行为
无机金属卤化物(IMHs)经常遭受严重的荧光热猝灭,限制了它们在高温下的应用。因此,研究具有抗热猝灭(ATQ)特性的IMHs具有重要的意义。在本研究中,我们开发了一条绿色合成路线,利用溶剂蒸发法成功合成了0D IMHs Cs2InCl5(H2O)。通过精确控制Sb3+、Yb3+和Er3+的掺杂比例,获得了独特的双模发射特性。随着温度的升高,化合物表现为下转换和上转换发光,相对灵敏度SR-max值分别为7.11% K-1和1.21% K-1。特别反常的是,该化合物在上转化过程中表现出非常规的ATQ行为。原位结构分析证实,在高温条件下,0D Cs2InCl5(H2O)金属卤化物经历了结构演变,转变为Cs3In2Cl9相,这是ATQ的原因。本研究为0D金属卤化物的ATQ异常提供了实验证据,为0D金属卤化物在高温感温和双模发光方面的多功能化提供了新的启发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
文献相关原料
公司名称
产品信息
阿拉丁
CsCl (cesium chloride)
阿拉丁
CsCl (cesium chloride)
阿拉丁
InCl3
阿拉丁
YbCl3
阿拉丁
ErCl3
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


