Nucleophilicity of the Nitrile N Atom Whose Lone Pair Is Blocked by Metal Coordination. The π-Hole Interaction between an Arene Carbon and the Metal-Bound Nitrile Nitrogen
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Cocrystallization of CuI with NCNMe2 in the presence of substituted perfluoroarenes─iodoperfluorobenzene (IFB), 4,4′-diiodoperfluorobiphenyl (4,4′-FIBP), and 4-bromoperfluorobenzonitrile (4-BrFBN)─led to the formation of three types of adducts 1·2(IFB), 1·4,4’FIBP, and 1·4-BrFBN (1 is Cu4I4(NCNMe2)4), all studied by X-ray crystallography. In these cocrystals, the coordinated nitrile N atom (whose electron pair is engaged in metal coordination) still acts as an electron donor, forming π-hole interactions, specifically, π-holearene···Nnitrile, with the perfluoroarenes. These interactions were examined in the context of their occurrence alongside other interactions involving C atoms of the electron-deficient aromatic rings and nucleophilic atoms of the copper cluster. Comprehensive theoretical calculations, including MEP, QTAIM/NCI plot analysis, EDA, ELF projections, and ETS-NOCV calculations, revealed that the nitrile ligand N atom maintains significant negative potential and that π-hole interactions are energetically more favorable than σ-hole interactions in the studied systems. This nucleophilicity is based on a noticeable contribution of the heterocumulene form, Cu–N–═C═N+Me2, in the resonance hybrid of Cu-bound NCNMe2: a phenomenon influenced by both the coordination and the conjugation between the NR2 and CN groups The discovery of π-holearene···Nnitrile contacts adds a new dimension to our understanding of coordinated push–pull nitriles, in particular dialkylcyanamides, revealing that the coordinated nitrile N atom can still function as a nucleophile in noncovalent binding.
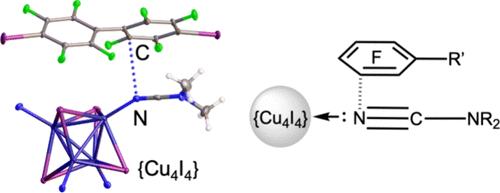
孤对被金属配位阻断的腈N原子的亲核性。芳烃碳与金属结合的腈氮之间的π孔相互作用
在取代的全氟芳烃──碘过氟苯(IFB)、4,4′-二碘过氟联苯(4,4′-FIBP)和4-溴过氟苯腈(4- brfbn)存在下,CuI与NCNMe2共结晶,形成了三种类型的加成物1·2(IFB)、1·4,4′-FIBP和1·4- brfbn(1为Cu4I4(NCNMe2)4),均通过x射线晶体学进行了研究。在这些共晶中,配位的腈N原子(其电子对参与金属配位)仍然充当电子给体,与全氟芳烃形成π-空穴相互作用,特别是π-孔芳烃···腈与全氟芳烃形成相互作用。这些相互作用是在它们与其他涉及缺电子芳香环的C原子和亲核铜簇原子的相互作用的背景下进行的。综合理论计算,包括MEP、QTAIM/NCI图分析、EDA、ELF预测和ETS-NOCV计算,表明在研究体系中,腈配体N原子保持了显著的负电位,且π-空穴相互作用在能量上比σ-空穴相互作用更有利。这种亲核性是基于在cu结合的NCNMe2的共振杂化中,异聚烯形式Cu-N - = CN +Me2的显著贡献:π-孔烯···腈键的发现为我们对推拉式配位腈特别是二烷基氰酰胺的认识提供了一个新的视角,揭示了配位的腈N原子在非共价结合中仍然可以作为亲核试剂发挥作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


