Insertion Behavior of Dialkylgermylene Ge(FluTMS)2 in Different E–X σ Bonds (E = Au, Si, Ge, Sn) and Its Relevance for Germanium Cluster Formation
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
We present the insertion behavior of the alkyl-substituted germylene Ge(FluTMS)2 in different E–X σ bonds (E = Au, Si, Ge, Sn), first with the isolation of the germylgold complex (FluTMS)2Ge(Cl)AuPPh3. Afterward, the oxidative addition of GeCl4 to Ge(FluTMS)2 gives the digermane (FluTMS)2Ge(Cl)GeCl3, followed by a reductive elimination of GeCl2 and the formation of the oxidation product (FluTMS)2GeCl2. A comparable behavior is observed, with the homologues ECl4 (E = Si, Sn) stopping at different steps of the reaction. The formation of the germylgermylene (FluTMS)2Ge(Cl)GeCl starting from Ge(FluTMS)2 and GeCl2·dioxane is also spectroscopically observable, followed by ligand rearrangements to the tetramer [FluTMSGeCl]4. Adding a phosphine as the Lewis base has a direct influence on the stability and structure of (FluTMS)2Ge(Cl)GeCl, leading to the formation of FluTMSGeCl·PEt3 as a stable product. Subsequent investigations of Ge(FluTMS)2 with a metastable Ge(I)Br solution show that at a ratio of 6:1 GeBr/Ge(FluTMS)2 the alkyl-substituted germylene no longer acts as a redox-active agent but as a cluster building block. This link and the involvement of a germylene in insertion reactions give an indication of how the construction or expansion of Ge cluster species can operate.
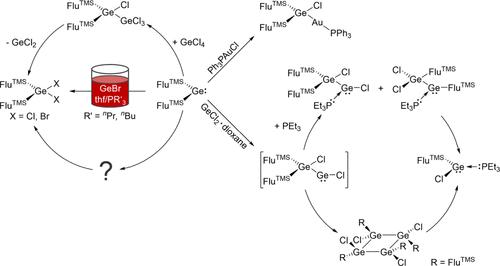
二基锗烯锗(FluTMS)2在不同E - x σ键(E = Au, Si, Ge, Sn)上的插入行为及其与锗团簇形成的关系
研究了烷基取代锗金(FluTMS)2在不同E - x σ键(E = Au, Si, Ge, Sn)上的插入行为,并首次分离出了锗金配合物(FluTMS)2Ge(Cl)AuPPh3。然后,将GeCl4氧化加入到Ge(FluTMS)2中,得到双草胺(FluTMS)2Ge(Cl)GeCl3,接着是GeCl2的还原消除,形成氧化产物(FluTMS)2GeCl2。类似的行为被观察到,与同系物ec14 (E = Si, Sn)停止在反应的不同步骤。从Ge(FluTMS)2和GeCl2·二恶烷开始的germylgermylene (FluTMS)2Ge(Cl)GeCl的形成也可以在光谱上观察到,随后是配体重排到四聚体[FluTMSGeCl]4。添加膦作为Lewis碱,直接影响(FluTMS)2Ge(Cl)GeCl的稳定性和结构,形成稳定产物FluTMSGeCl·PEt3。随后对Ge(FluTMS)2与亚稳Ge(I)Br溶液的研究表明,当GeBr/Ge(FluTMS)2的比例为6:1时,烷基取代的germylene不再作为氧化还原活性剂,而是作为簇的构建块。这种联系以及插入反应中二甲苯的参与表明了Ge簇的构建或扩展是如何运作的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


