Effect of a Combination of Amino Acids on the Kinetics and Separation of a Biogas Mixture through Gas Hydrate Technology
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
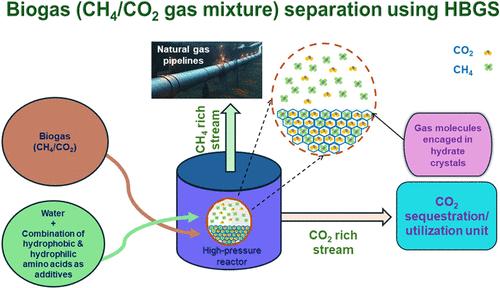
Hydrate-based gas separation has the potential to capture and separate CO2 in biogas in order to enrich its calorific value. Authors’ previous work examined the individual effects of hydrophobic and hydrophilic amino acids on the hydrate formation kinetics by employing biogas. The present study examines the kinetics of hydrate formation by employing a CH4(50%)/CO2(50%) gas mixture using the combination of two categories of amino acids at varying concentration ratios. Such a combination effect of different amino acids on the hydrate formation kinetics has not been examined before. In this article, kinetic parameters such as CO2 recovery, separation factor, gas uptake, t90, residual, and hydrate gas composition have been calculated and presented along with morphological observations during hydrate formation. Different combinations of hydrophobic (l-methionine, l-tryptophan) and hydrophilic amino acids (l-arginine, l-histidine) have been used at the experimental conditions of 274.2 K and 6.0 MPa in different concentration ratios. The highest recovery of CO2 of 42% was achieved using 0.1 wt % l-tryptophan and 0.1 wt % l-histidine in 1:1 concentration ratio, while the highest gas uptake of 133 ± 1.6 mmol/mol was achieved using 0.1 wt % l-tryptophan and 0.3 wt % l-histidine (1:3 concentration ratio). The hydrate dissociation kinetics was also examined along with morphological observations in the study.
氨基酸组合对沼气水合物分离动力学的影响
水合物基气体分离技术有潜力捕获和分离沼气中的二氧化碳,以丰富其热值。作者以前的工作考察了疏水和亲水氨基酸对利用沼气形成水合物动力学的个别影响。本研究通过使用CH4(50%)/CO2(50%)气体混合物,使用不同浓度比的两类氨基酸的组合,研究了水合物形成的动力学。不同氨基酸对水合物形成动力学的这种组合效应以前还没有研究过。本文计算并给出了水合物形成过程中的动力学参数,如CO2回收率、分离因子、气体吸收量、t90、残余量和水合物气体组成。在274.2 K和6.0 MPa的实验条件下,以不同的浓度比,使用了疏水氨基酸(l-蛋氨酸、l-色氨酸)和亲水氨基酸(l-精氨酸、l-组氨酸)的不同组合。以0.1 wt %的l-色氨酸和0.1 wt %的l-组氨酸为1:1的浓度比,CO2回收率最高,为42%;以0.1 wt %的l-色氨酸和0.3 wt %的l-组氨酸为1:3的浓度比,CO2吸收率最高,为133±1.6 mmol/mol。水合物解离动力学和形态观察也在研究中进行了检验。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


