DFT Studies on the Influence of the Substituted Central Atom on Molybdenum-Based Heteropolyanions Used for the Catalytic Oxidation of Methacrolein to Methacrylic Acid
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Heteropolyacids have been used as efficient and sustainable catalysts for the selective oxidation of methacrolein (MAL) to methacrylic acid (MAA) in recent years due to their adjustable structure. Herein, the influence of the substituted central atom (X = Al, Ga, Si, Ge, As, and S) on the structural and electronic properties of primary molybdenum-based heteropolyanions was studied. The density functional theory results indicated that the bridge oxygen of the heteropolyanion was more prone to breakage than the terminal oxygen. The interaction between MAL and catalytic sites of the molybdenum-based heteropolyanion having different central atoms and the corresponding adsorption energies were calculated. The reaction mechanism of MAL oxidation catalyzed by the heteropolyanion was systematically studied, which included the activation of the carbonyl group, hydrogen transfer, and generation of ester-based intermediates, formation of a carboxyl structure, and oxygen activation. The first step of carbonyl group activation is confirmed as the rate-determining step of this reaction. As a result, a linear relationship was discovered between the activation barrier of the rate-determining step and the lowest unoccupied molecular orbital (LUMO) of the heteropolyanion as the LUMO energy reflected the redox potential, which indicated that an increase in the LUMO energy would lead to poor catalytic performance. In addition, the activation energy of the rate-determining step would decrease in positive proportion to the ratio of the negative charge of the heteropolyanion to the electronegativity of the central atom. This correlation result indicated that the reactivity of the heteropolyanion could be predicted by calculating the negative charge and electronegativity. These findings would provide theoretical insights into the heteropolyanion at the molecular level and assist in the modification of the heteropolyacid structure to improve the catalytic performance.
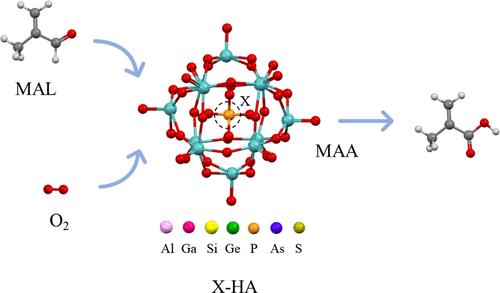
取代中心原子对甲基丙烯醛催化氧化制甲基丙烯酸用钼基杂多阴离子影响的DFT研究
杂多酸由于其结构可调,近年来被用作甲基丙烯醛(MAL)选择性氧化制甲基丙烯酸(MAA)的高效、可持续催化剂。本文研究了取代中心原子(X = Al, Ga, Si, Ge, As和S)对钼基原生杂多阴离子结构和电子性能的影响。密度泛函理论结果表明,异多阴离子的桥氧比末端氧更容易断裂。计算了具有不同中心原子的钼基杂多阴离子的MAL与催化位点的相互作用及相应的吸附能。系统研究了杂多阴离子催化MAL氧化的反应机理,包括羰基活化、氢转移、酯基中间体的生成、羧基结构的形成、氧活化等。羰基活化的第一步被确定为该反应的速率决定步骤。结果发现,决定速率步骤的激活势垒与杂多阴离子的最低未占据分子轨道(LUMO)呈线性关系,因为LUMO能量反映了氧化还原电位,这表明LUMO能量的增加会导致催化性能变差。此外,速率决定步骤的活化能将与异多阴离子的负电荷与中心原子的电负性之比成正比例地降低。这一关联结果表明,可以通过计算负电荷和电负性来预测异多阴离子的反应性。这些发现将在分子水平上为研究杂多酸提供理论依据,并有助于对杂多酸结构进行修饰以提高催化性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


