Halogenated bisphenol F compounds: Chlorination-mediated formation and photochemical fate in sunlit surface water
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
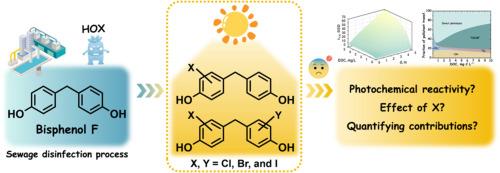
Halogenated bisphenol compounds are prevalent in urban water systems and may pose greater environmental risks than their bisphenol precursors. This study explored the formation of halogenated bisphenol F (BPF) in water chlorination and their subsequent transformation behaviors in receiving waters. The kinetics and pathways of BPF halogenation with chlorine, bromine, and iodine were firstly investigated. BPF chlorination followed second-order kinetics, with pH-dependent second-order rate constants (kapp) ranging from 1.0 M−1 s−1 at pH 5.0 to 50.4 M−1 s−1 at pH 9.0. The kapp of BPF with bromine and iodine were 4−5 orders of magnitude higher than those of chlorine. The degradation potential of halogenated BPF products in sunlit surface waters was also evaluated, focusing on both direct and indirect photolysis. Indirect photolysis, involving reactions with excited triplet state of CDOM (3CDOM*), •OH and 1O2, emerged as the primary degradation pathway for BPF, while both direct photolysis and indirect photolysis with 3CDOM* predominated for mono- and dihalogenated BPF products. Compared with BPF, the photodegradation of halogenated products was significantly enhanced. Photolysis experiments in wastewater-receiving wetland water demonstrated effective degradation of halogenated BPF products, highlighting the pivotal role of sunlight in their environmental fate. Overall, this study advances understanding of the formation and fate of halogenated BPF products and provides valuable insights for managing the environmental impacts of these emerging contaminants.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


