Competitive Adsorption of Arsenate and Phosphate on Hematite Facets: Molecular Insights for Enhanced Arsenic Retention
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
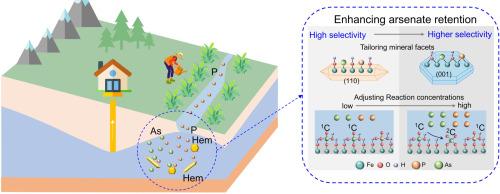
Understanding the competition for adsorption between arsenate and other common oxyanions at mineral-water interfaces is critical for enhancing arsenate retention in the subsurface environment and mitigating exposure risks. This study investigated the competitive adsorption between arsenate and phosphate on hematite facets using batch experiments, together with in-situ infrared spectroscopy, two-dimensional correlation spectroscopy (2D-COS), and ab initio molecular dynamic (AIMD) simulations. This study's findings revealed that hematite exhibited notable selectivity for arsenate over phosphate in both adsorption capacity and rate, with selectivity significantly influenced by the exposed facets of the hematite and reaction concentrations. To wit, the (001) facet exhibited stronger selectivity for arsenate than the (110) facet, and increasing reaction concentration further enhances this selectivity. This selectivity was driven by surface hydroxy structure-mediated complexation, where both surfaces primarily formed stable inner-sphere monodentate complexes with an affinity for arsenate. On the (001) surface, the available Fe2OH featured two close-spaced iron sites (Fe - Fe ≈ 2.86Å), enabling arsenate to interact with both sites simultaneously, significantly boosting arsenate selectivity. At higher surface loadings, the (110) surface formed partially more selective bidentate binuclear complexes, further enhancing arsenate retention. These findings emphasize the critical role of interfacial complexation, particularly the formation of inner-sphere bidentate complexes and the availability of iron sites, in controlling arsenate retention. By tailoring mineral facets and optimizing reaction conditions to improve iron site availability and promote bidentate complexation, arsenate retention can be significantly enhanced in phosphate-rich aquatic environments, such as rivers and groundwater in agricultural areas.
Fe2OH featured two close-spaced iron sites (Fe - Fe ≈ 2.86Å), enabling arsenate to interact with both sites simultaneously, significantly boosting arsenate selectivity. At higher surface loadings, the (110) surface formed partially more selective bidentate binuclear complexes, further enhancing arsenate retention. These findings emphasize the critical role of interfacial complexation, particularly the formation of inner-sphere bidentate complexes and the availability of iron sites, in controlling arsenate retention. By tailoring mineral facets and optimizing reaction conditions to improve iron site availability and promote bidentate complexation, arsenate retention can be significantly enhanced in phosphate-rich aquatic environments, such as rivers and groundwater in agricultural areas.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


