Degradation of polyamide nanofiltration membranes by free chlorine and halide ions: kinetics, mechanisms, and implications
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
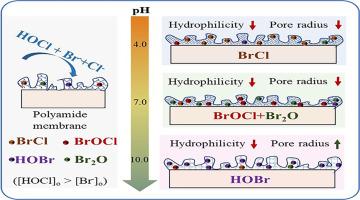
The kinetics of polyamide membrane degradation by free chlorine and halide ions (Br− and Cl−) were innovatively evaluated based on physicochemical properties and filtration performance, using water/solute permeability coefficient in addition to bromide incorporation as important indicators. The reaction rate constants for the reduced water and H3BO3 permeability coefficient were 1-2 orders of magnitude higher at 0–1 h than 1-10 h. N-bromination and bromination-promoted hydrolysis are dominant degradation mechanisms at 0−1 h (reflected by the breakage of hydrogen bond, the increased Ca binding content, and the increased charge density), and ring-bromination further occurs at 1−10 h (reflected by the disappearance or weakening of aromatic amide band and the nearly constant hydrogen bond). The more reactive but less abundant brominating agents (Br2O, BrOCl, BrCl, and Br2) played significant roles in membrane degradation, contradicting the conventional belief that HOBr is the only reactive species. BrCl at pH 4.0 and BrOCl and Br2O at pH 7.0 made significantly higher contributions to membrane degradation than HOBr (>76% vs. <13%). The increased contribution of BrCl and Br2 with the increased [Cl−] and [Br−]ex (the excess bromide, defined as [Br−]o − [HOCl]o when [Br−]o > [HOCl]o), respectively, was responsible for the greater reduction of water permeability coefficient. The innovative and simple approach developed in this study provides important insights to evaluate and predict membrane degradation.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


