Multistage Generation Mechanisms of Reactive Oxygen Species and Reactive Chlorine Species in a Synergistic System of Anodic Oxidation Coupled with in Situ Free Chlorine and H2O2 Production
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Electro-oxidation (EO) is an efficient approach to removing refractory organics in wastewater. However, the interference from chlorine ions (Cl–) can generate reactive chlorine species (RCS), potentially leading to the production of undesirable chlorinated byproducts. A novel approach involving the cathodic oxygen reduction reaction (ORR) for in situ H2O2 production has emerged as a promising strategy to counteract this issue. This study systematically investigated the dynamics and transformation of RCS and reactive oxygen species (ROS) in an ORR/chloride-containing EO (EO-Cl) system, elucidating their respective roles in organic removal and chlorinated byproduct minimization. Distinct generation rates and patterns were observed for free chlorine and H2O2 in the ORR/EO-Cl system. The rapid generation of free chlorine at the anode quickly reached a dynamic equilibrium, which contrasted with the moderate, continuous cathodic production of H2O2, resulting in considerable H2O2 accumulation over time. This difference established kinetics-driven ROS and RCS formation and distribution, influencing the subsequent organic degradation process. Three distinct stages were identified in the degradation process. In stage I, free chlorine was the primary species, along with reactive species including Cl2•–, 1O2, ClO•, HO•, and Cl•. In stage II, the gradual accumulation of H2O2 consumed free chlorine, favoring the formation of 1O2 and HO•. In stage III, excessive H2O2 quenched the free radicals. Insights into these multistage mechanisms reveal that the rapid degradation of chlorinated byproducts by 1O2 and HO• occurs in stage II of the ORR/EO-Cl system.
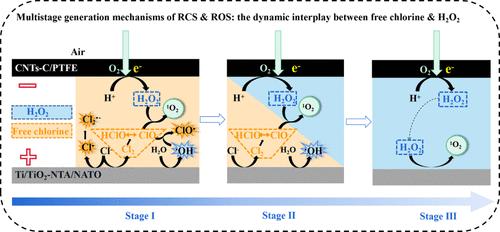
阳极氧化-原位游离氯- H2O2协同系统中活性氧和活性氯的多级生成机理
电氧化法是去除废水中难降解有机物的有效方法。然而,氯离子(Cl -)的干扰可以产生活性氯(RCS),可能导致产生不希望的氯化副产物。利用阴极氧还原反应(ORR)原位生产H2O2的新方法有望解决这一问题。本研究系统地研究了RCS和活性氧(ROS)在ORR/含氯EO (EO- cl)体系中的动态和转化,阐明了它们各自在有机去除和氯化副产物最小化中的作用。在ORR/EO-Cl体系中,游离氯和H2O2的生成速率和模式不同。阳极快速生成的游离氯很快达到动态平衡,这与缓慢、连续的阴极生成H2O2形成对比,随着时间的推移,H2O2积累相当大。这种差异建立了动力学驱动的ROS和RCS的形成和分布,影响了随后的有机降解过程。降解过程分为三个阶段。在第一阶段,游离氯是主要物质,其次是Cl2•-、1O2、ClO•、HO•和Cl•等活性物质。在第II阶段,H2O2的逐渐积累消耗了游离氯,有利于形成1O2和HO•。在第三阶段,过量的H2O2淬灭了自由基。对这些多阶段机制的深入研究表明,1O2和HO•对氯化副产物的快速降解发生在ORR/EO-Cl系统的第II阶段。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


