Visible-light-driven aldehyde ammoxidation to nitrile via •O2– and •NHx radicals generation over Ni(OH)2/TiO2 p-n heterojunctions
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Aldehydes ammoxidation is a green and promising route for selective synthesis of nitrile, but developing low-cost and efficient catalytic system under mild condition remains a challenge. Herein, we explore a novel catalytic system where the designed Ni(OH)2/TiO2 p-n heterojunction catalyst could efficiently drive aldehydes ammoxidation with NH3·H2O and O2 at ambient temperature and pressure by using visible-light as the only energy input. The superoxide (•O2–) generated by reducing O2 with photogenerated electrons and amino radicals (•NHx) produced by oxidizing NH3·H2O with photogenerated holes, are successfully identified as key species for the formation of nitriles. Meanwhile, •NHx and •O2– radicals individually work on the formation of the intermediate aldimine intermediate and its oxidation to nitrile. The performance of the engineered Ni(OH)2/TiO2 p-n heterojunctions in the reaction process is much more superior to other catalyst systems. This study develops nitrile synthesis route under mild conditions and present new opportunities for constructing low-cost photocatalyst for chemical synthesis. It also provides a better understanding of the radical species and how they work in aldehydes ammoxidation reactions.
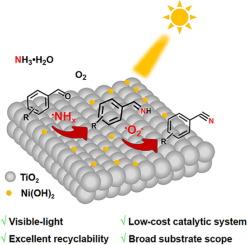

在Ni(OH)2/TiO2 p-n异质结上通过•O2 -和•NHx自由基生成的可见光驱动醛氨氧化制腈
醛类氨氧化是一种绿色、有前途的选择性合成腈的途径,但在温和条件下开发低成本、高效的催化体系仍是一个挑战。在此,我们探索了一种新的催化体系,其中设计的Ni(OH)2/TiO2 p-n异质结催化剂可以在室温和常压下以可见光作为唯一的能量输入,有效地驱动醛类与NH3·H2O和O2的氨氧化反应。利用光生电子还原O2生成的超氧化物(•O2 -)和利用光生空穴氧化NH3·H2O生成的氨基自由基(•NHx)被成功地鉴定为腈形成的关键物质。同时,•NHx和•O2 -自由基分别作用于中间醛胺中间体的形成及其氧化成腈。工程制备的Ni(OH)2/TiO2 p-n异质结在反应过程中的性能远远优于其他催化剂体系。本研究开辟了温和条件下的腈合成路线,为构建低成本的化学合成光催化剂提供了新的机会。它也提供了一个更好的理解自由基种类和它们如何在醛氨氧化反应中起作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


