KBTBD8/RRP15 as a potential novel therapeutic target associates with lenvatinib-inhibited progression in hepatocellular carcinoma both in vitro and in vivo
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
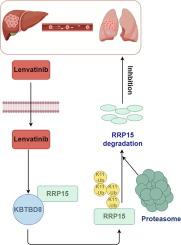
We have previously demonstrated that RRP15 (Ribosomal RNA Processing 15 Homolog) was significantly elevated in hepatocellular carcinoma (HCC) and correlated directly with poor prognosis. RRP15 suppression curtails HCC progression through induction of cellular senescence and apoptosis. However, the impact of RRP15 on the precise therapeutic potential of lenvatinib has remained underexplored.
Objective
To investigate the relationship between RRP15 expression and sensitivity of lenvatinib in HCC treatment, and also explore the potential of targeting RRP15 by lenvatinib to inhibit HCC progression.
Methods
RRP15 and KBTBD8 (Kelch Repeat and BTB Domain Containing 8) expression was examined using western blot and immunohistochemistry. Cell viability, proliferation, migration and invasion as well as apoptosis were assessed using CCK-8, clonogenic assays, transwell, TUNEL (Terminal Deoxynucleotidyl Transferase mediated dUTP Nick-End Labeling) and Annexin V staining assays. The interaction between RRP15 and KBTBD8 was identified through pull-down and mass spectrometry analysis and further validated by immunofluorescence and co-immunoprecipitation assays. RRP15 ubiquitination and degradation were assessed using cycloheximide treatment, plasmid transfection and co-immunoprecipitation, followed by western blot analysis. Tail vein injection lung metastasis model was performed to determine tumor metastasis in vivo.
Results
We reveled a correlation between RRP15 downregulation and enhanced sensitivity to lenvatinib, presenting marked suppression of metastasis and invasiveness. Proteomic analyses and subsequent validation disclosed the pivotal role of the E3 ubiquitin ligase KBTBD8 in mediating the ubiquitination and subsequent degradation of RRP15 protein post-lenvatinib treatment in HCC cells. KBTBD8 inhibition stalled RRP15 ubiquitination and degradation, while its overexpression accelerated these processes. Moreover, RRP15 overexpression fosters HCC cell proliferation and metastasis, a pathological effect mitigated by KBTBD8 overexpression. In vivo experiments further validate the role of lenvatinib in promoting RRP15 degradation via KBTBD8 upregulation.
Conclusions
Our study elucidated a previously unidentified mechanism of lenvatinib action and identified the RRP15-KBTBD8 axis as a novel therapeutic target in HCC, offering new avenues for treatment strategies in combating HCC.
KBTBD8/RRP15作为一种潜在的新型治疗靶点与lenvatinib在体外和体内抑制肝细胞癌的进展有关
我们之前已经证明RRP15(核糖体RNA加工15同源物)在肝细胞癌(HCC)中显著升高,并与不良预后直接相关。抑制RRP15通过诱导细胞衰老和凋亡来抑制HCC的进展。然而,RRP15对lenvatinib精确治疗潜力的影响仍未得到充分探讨。目的探讨lenvatinib在HCC治疗中RRP15表达与敏感性的关系,探讨lenvatinib靶向RRP15抑制HCC进展的潜力。方法采用western blot和免疫组化方法检测srrp15和KBTBD8 (Kelch Repeat和BTB Domain Containing 8)的表达。采用CCK-8、克隆实验、transwell、TUNEL(末端脱氧核苷酸转移酶介导的dUTP镍端标记)和Annexin V染色法评估细胞活力、增殖、迁移、侵袭和凋亡。RRP15和KBTBD8的相互作用通过拉下和质谱分析确定,并通过免疫荧光和共免疫沉淀试验进一步验证。采用环己亚胺处理、质粒转染和免疫共沉淀法评估RRP15的泛素化和降解,然后进行western blot分析。建立尾静脉注射肺转移模型,观察肿瘤在体内的转移情况。结果我们发现RRP15下调与lenvatinib敏感性增强之间存在相关性,表现出明显的转移和侵袭性抑制。蛋白质组学分析和随后的验证揭示了E3泛素连接酶KBTBD8在lenvatinib治疗后介导HCC细胞中RRP15蛋白的泛素化和随后的降解中的关键作用。KBTBD8抑制抑制了RRP15的泛素化和降解,而其过表达则加速了这一过程。此外,RRP15的过表达促进了HCC细胞的增殖和转移,而KBTBD8的过表达减轻了这一病理作用。体内实验进一步验证lenvatinib通过上调KBTBD8促进RRP15降解的作用。结论我们的研究阐明了lenvatinib的作用机制,并确定了RRP15-KBTBD8轴是HCC的一个新的治疗靶点,为HCC的治疗策略提供了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


