Macrophage P2Y12 regulates iron transport and its inhibition protects against atherosclerosis
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Iron retention is commonly observed in atherosclerotic plaques and is believed to be detrimental to atherosclerosis. Platelet P2Y12 is a target of antiplatelet therapy in preventing thrombotic complications of atherosclerosis. The protective effect of P2Y12 on hematopoiesis reported by our previous work implies the involvement of P2Y12 in iron metabolism.
Objectives
This study further investigated the role of P2Y12 in the iron metabolism of macrophages, the key player in systemic iron homeostasis and atherosclerosis.
Methods
The association between serum iron and the use of P2Y12 inhibitors was evaluated by a case-control study in human. Secondary iron overload and atherosclerosis animal models were established in P2Y12-deficient zebrafish to explore the role of P2Y12 in macrophage iron metabolism in vivo. Both iron-overloaded murine primary peritoneal macrophages (PMs) and ox-LDL–treated PMs with P2Y12 knockdown were used for in vitro studies. RNA sequencing and pharmacological approaches were performed to investigate the downstream mechanisms.
Results
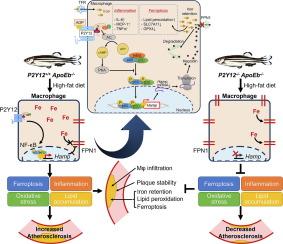
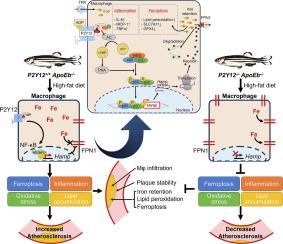
Increased serum iron level was positively associated with P2Y12 inhibitor usage [odds ratio (OR) = 10.333 (1.281–83.370)]. Elevated serum iron level and transferrin saturation, reduced hepatic and splenic iron content, and decreased iron staining in macrophages were observed in secondary iron overload P2Y12-deficient zebrafish. Deficiency of P2Y12 in ApoEb-/- zebrafish fed a high-fat diet reduced atherosclerosis progression and intraplaque iron retention. Furthermore, reduced ferritin, restored cell viability and expression of ferroptosis marker proteins, and decreased ROS formation and inflammatory cytokines were observed in both iron-overloaded and ox-LDL–treated PMs with P2Y12 knockdown in vitro, while reversed phenotypes were observed after agonist-induced P2Y12 activation. Mechanistically, P2Y12 inhibition in iron-overloaded or ox-LDL–treated PMs suppressed NF-κB p65 phosphorylation and hepcidin expression, both of which were reversed by P2Y12 activation.
Conclusion
P2Y12 inhibition decreased hepcidin autocrine through repressing NF-κB p65 phosphorylation in macrophages, preventing intracellular iron retention and atherosclerosis.
巨噬细胞P2Y12调节铁转运及其抑制对动脉粥样硬化的保护作用
铁潴留常见于动脉粥样硬化斑块,并被认为对动脉粥样硬化有害。血小板P2Y12是抗血小板治疗预防动脉粥样硬化血栓性并发症的靶点。我们之前报道的P2Y12对造血的保护作用提示P2Y12参与铁代谢。目的进一步探讨P2Y12在巨噬细胞铁代谢中的作用,巨噬细胞是全身铁稳态和动脉粥样硬化的关键。方法通过病例对照研究,评价血清铁与P2Y12抑制剂使用的关系。在P2Y12缺陷斑马鱼中建立继发性铁超载和动脉粥样硬化动物模型,探讨P2Y12在体内巨噬细胞铁代谢中的作用。铁超载小鼠原代腹腔巨噬细胞(PMs)和氧低密度脂蛋白处理的P2Y12敲低小鼠腹腔巨噬细胞被用于体外研究。采用RNA测序和药理学方法研究其下游机制。结果血清铁水平升高与P2Y12抑制剂使用呈正相关[比值比(OR) = 10.333(1.281-83.370)]。继发性铁超载p2y12缺陷斑马鱼血清铁水平和转铁蛋白饱和度升高,肝脏和脾脏铁含量降低,巨噬细胞铁染色降低。高脂饲料喂养的ApoEb-/-斑马鱼缺乏P2Y12可减少动脉粥样硬化进展和斑块内铁潴留。此外,在体外实验中,铁超载和ox- ldl处理的P2Y12敲除的pmms均观察到铁蛋白含量降低,细胞活力和凋亡标记蛋白表达恢复,ROS形成和炎症细胞因子减少,而在激动剂诱导的P2Y12激活后,观察到表型逆转。从机制上讲,铁超载或ox- ldl处理的pm中P2Y12的抑制抑制了NF-κB p65的磷酸化和hepcidin的表达,这两种情况都被P2Y12激活逆转。结论p2y12抑制可通过抑制巨噬细胞NF-κB p65磷酸化降低hepcidin自分泌,防止细胞内铁潴留和动脉粥样硬化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


