Insights into the separation, enantioseparation and recognition mechanisms of methamphetamine isotopologues on achiral and polysaccharide-based chiral columns in high-performance liquid chromatography
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Background
Isotopologues resulting from the labelling of molecules with deuterium have attracted interest due to the isotope effect observed in chemistry and biosciences. Isotope effect may also play out in noncovalent interactions and mechanisms leading to intermolecular recognition. In chromatography, differences in retention time between isotopologues, as well as between isotopomers have been observed resulting in two different elution sequences (isotope effects): the normal isotope effect when heavier isotopologues retain longer than lighter analogues, and the inverse isotope effect featuring the opposite elution order. Although several cases of deuterium isotope effects have been reported so far, the molecular bases of these phenomena remain unclear.
Results
We report the separation of isotopologues of methamphetamine (N-methyl-1-phenylpropan-2-amine) (MET), featuring different number and location of deuterium substituents, on an achiral column by high-performance liquid chromatography (HPLC), as well as the simultaneous separation of isotopologues and their enantiomers on some polysaccharide-based chiral columns. The effects of the number and location of deuterium substituents introduced in MET's structure, of the surface chemistry of the adsorbent, and the mobile phase composition and pH on the retention and separation of isotopologues and their enantiomers were examined. In several cases, the effect of temperature was also evaluated, and the thermodynamic quantities associated with isotopologue adsorption, separation, and enantioseparation were also calculated. To elucidate the molecular bases of the experimental observations, quantum mechanics calculations were performed focusing on the vibrational degree of freedom calculated for models of isotopologue-selector complexes. On this basis, zero-point vibrational energies were computed as useful descriptors to differentiate computationally between deuterated isotopologues.
Significance
Using HPLC as experimental technique with the following dual function: 1) separation of MET isotopologues and their enantiomers; 2) exploring noncovalent interactions underlying their separation. The degree of deuteration, location of deuterium substituents, and mobile phase pH play key roles in isotopologue separations. By integrating experimental and computational analyses, noncovalent interactions underlying normal and inverse isotope effects were deconvoluted, highlighting the contribution of hydrogen bond, hydrophobic and dispersive forces in isotopologue and enantiomer separation.
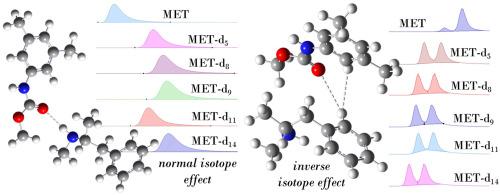
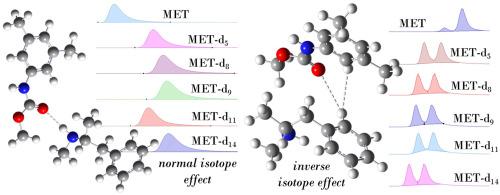
甲基苯丙胺同位素在高效液相色谱非手性柱和多糖手性柱上的分离、对映体分离和识别机制研究
由于在化学和生物科学中观察到同位素效应,用氘标记分子所产生的异拓扑物引起了人们的兴趣。同位素效应也可能在导致分子间识别的非共价相互作用和机制中发挥作用。在色谱中,同位素物之间以及同位素物之间的保留时间差异导致了两种不同的洗脱顺序(同位素效应):重同位素物比轻类似物保留时间长的正常同位素效应,以及相反洗脱顺序的逆同位素效应。虽然到目前为止已经报道了几个氘同位素效应的案例,但这些现象的分子基础仍不清楚。结果采用高效液相色谱法在非手性色谱柱上分离出了氘取代基数目和位置不同的甲基苯丙胺(n-甲基-1-苯基丙烷-2-胺)的同位素物,并在一些基于多糖的手性色谱柱上同时分离出了同位素物及其对映体。考察了MET结构中引入氘取代基的数量和位置、吸附剂的表面化学性质、流动相组成和pH对同位素物及其对映体的保留和分离的影响。在一些情况下,还评估了温度的影响,并计算了与同位素吸附、分离和对映体分离相关的热力学量。为了阐明实验观察的分子基础,对同位素选择器配合物模型的振动自由度进行了量子力学计算。在此基础上,计算零点振动能作为区分氘化同位素的有用描述符。利用高效液相色谱作为实验技术具有以下双重功能:1)分离MET同位素物及其对映体;2)探索它们分离背后的非共价相互作用。氘化程度、氘取代基位置和流动相pH是影响同位素分离的关键因素。通过综合实验和计算分析,解开了正常和逆同位素效应下的非共价相互作用,突出了氢键、疏水和色散力在同位素和对映体分离中的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


