Central control of dynamic gene circuits governs T cell rest and activation
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
The ability of cells to maintain distinct identities and respond to transient environmental signals requires tightly controlled regulation of gene networks1–3. These dynamic regulatory circuits that respond to extracellular cues in primary human cells remain poorly defined. The need for context-dependent regulation is prominent in T cells, where distinct lineages must respond to diverse signals to mount effective immune responses and maintain homeostasis4–8. Here we performed CRISPR screens in multiple primary human CD4+ T cell contexts to identify regulators that control expression of IL-2Rα, a canonical marker of T cell activation transiently expressed by pro-inflammatory effector T cells and constitutively expressed by anti-inflammatory regulatory T cells where it is required for fitness9–11. Approximately 90% of identified regulators of IL-2Rα had effects that varied across cell types and/or stimulation states, including a subset that even had opposite effects across conditions. Using single-cell transcriptomics after pooled perturbation of context-specific screen hits, we characterized specific factors as regulators of overall rest or activation and constructed state-specific regulatory networks. MED12 — a component of the Mediator complex — serves as a dynamic orchestrator of key regulators, controlling expression of distinct sets of regulators in different T cell contexts. Immunoprecipitation–mass spectrometry revealed that MED12 interacts with the histone methylating COMPASS complex. MED12 was required for histone methylation and expression of genes encoding key context-specific regulators, including the rest maintenance factor KLF2 and the versatile regulator MYC. CRISPR ablation of MED12 blunted the cell-state transitions between rest and activation and protected from activation-induced cell death. Overall, this work leverages CRISPR screens performed across conditions to define dynamic gene circuits required to establish resting and activated T cell states. Resting and activated T cell states are established by context-specific regulators and dynamic gene circuits.
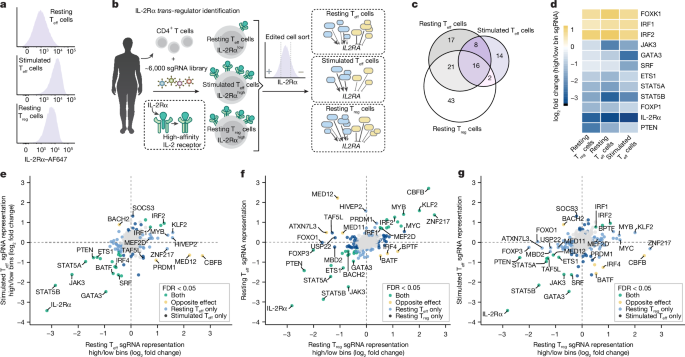

动态基因回路的中央控制控制T细胞的休息和激活
细胞维持独特身份和响应瞬时环境信号的能力需要基因网络的严格控制调控1,2,3。在人类原代细胞中,这些对细胞外信号作出反应的动态调节回路仍然不清楚。在T细胞中,上下文依赖性调节的需求是突出的,其中不同的谱系必须响应不同的信号来建立有效的免疫反应并维持稳态4,5,6,7,8。本研究中,我们在多个原代人CD4+ T细胞环境中进行了CRISPR筛选,以确定控制IL-2Rα表达的调节因子,IL-2Rα是促炎效应T细胞短暂表达的T细胞活化的典型标记物,在抗炎调节T细胞中组成表达,它是健康所必需的9,10,11。大约90%的IL-2Rα调节因子在不同的细胞类型和/或刺激状态下具有不同的作用,包括一个子集在不同的条件下甚至具有相反的作用。利用单细胞转录组学,我们将特定因素描述为整体休息或激活的调节因子,并构建了特定状态的调节网络。MED12是Mediator复合体的一个组成部分,作为关键调节因子的动态协调者,在不同的T细胞环境中控制不同调节因子的表达。免疫沉淀-质谱分析显示MED12与组蛋白甲基化COMPASS复合物相互作用。MED12是组蛋白甲基化和编码关键环境特异性调节因子的基因表达所必需的,包括休息维持因子KLF2和多功能调节因子MYC。CRISPR消融MED12减弱了细胞状态从静止到激活之间的转变,并保护细胞免受激活诱导的细胞死亡。总的来说,这项工作利用在不同条件下进行的CRISPR筛选来定义建立静息和激活T细胞状态所需的动态基因回路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


