Structure and assembly of the dystrophin glycoprotein complex
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
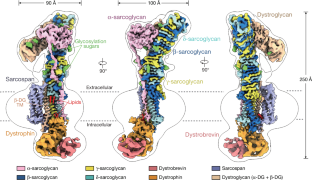
The dystrophin glycoprotein complex (DGC) has a crucial role in maintaining cell membrane stability and integrity by connecting the intracellular cytoskeleton with the surrounding extracellular matrix1–3. Dysfunction of dystrophin and its associated proteins results in muscular dystrophy, a disorder characterized by progressive muscle weakness and degeneration4,5. Despite the important roles of the DGC in physiology and pathology, its structural details remain largely unknown, hindering a comprehensive understanding of its assembly and function. Here we isolated the native DGC from mouse skeletal muscle and obtained its high-resolution structure. Our findings unveil a markedly divergent structure from the previous model of DGC assembly. Specifically, on the extracellular side, β-, γ- and δ-sarcoglycans co-fold to form a specialized, extracellular tower-like structure, which has a central role in complex assembly by providing binding sites for α-sarcoglycan and dystroglycan. In the transmembrane region, sarcoglycans and sarcospan flank and stabilize the single transmembrane helix of dystroglycan, rather than forming a subcomplex as previously proposed6–8. On the intracellular side, sarcoglycans and dystroglycan engage in assembly with the dystrophin–dystrobrevin subcomplex through extensive interaction with the ZZ domain of dystrophin. Collectively, these findings enhance our understanding of the structural linkage across the cell membrane and provide a foundation for the molecular interpretation of many muscular dystrophy-related mutations. A structural study of native dystrophin glycoprotein complex from mouse skeletal muscle reveals an extended tower-like architecture that provides multiple binding sites on both sides of the membrane for signalling and effector molecules, reshaping our understanding of how the complex is assembled.

肌营养不良蛋白糖蛋白复合物的结构和组装
肌营养不良蛋白糖蛋白复合物(DGC)通过连接细胞内细胞骨架和周围的细胞外基质,在维持细胞膜稳定性和完整性方面起着至关重要的作用1,2,3。肌营养不良蛋白及其相关蛋白的功能障碍导致肌肉营养不良,这是一种以进行性肌肉无力和变性为特征的疾病4,5。尽管DGC在生理和病理中发挥着重要作用,但其结构细节在很大程度上仍然未知,阻碍了对其组装和功能的全面了解。我们从小鼠骨骼肌中分离了天然DGC,获得了其高分辨率结构。我们的发现揭示了一个明显不同的结构,从以前的模型DGC组装。具体来说,在细胞外,β-、γ-和δ-肌聚糖共同折叠形成一个特化的细胞外塔状结构,它通过为α-肌聚糖和糖酐提供结合位点,在复杂的组装中起着核心作用。在跨膜区域,肌聚糖和肌跨包围并稳定了单跨膜螺旋的肌聚糖,而不是像之前提出的那样形成亚复合物6,7,8。在细胞内,肌聚糖和肌营养不良聚糖通过与肌营养不良蛋白的ZZ结构域的广泛相互作用,与肌营养不良蛋白-肌营养不良蛋白亚复合物进行组装。总的来说,这些发现增强了我们对细胞膜结构联系的理解,并为许多肌营养不良相关突变的分子解释提供了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


