Nanozyme as Tumor Energy Homeostasis Disruptor to Augment Cascade Catalytic Therapy
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
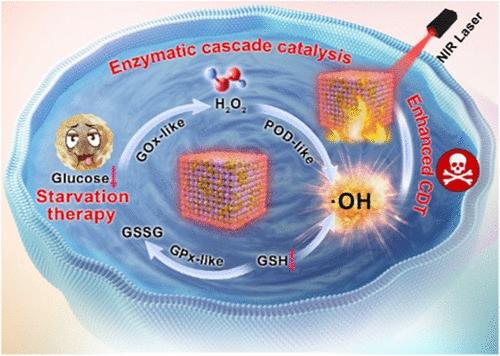
Breaking the balance of the tumor microenvironment and reshaping it sustainably remain major challenges in lung cancer treatment. Here, a “tumor energy homeostasis disruptor”, the Cu2O@Au nanozyme was developed, which exhibits excellent glucose oxidase-like activity, enabling it to be used for starvation therapy and as a mimic peroxidase for chemodynamic therapy (CDT), producing •OH. Cu2O@Au nanozymes consume glucose at the tumor site to block the tumor’s energy supply, produce H2O2 continuously, and lower the pH to enhance the efficiency of CDT, initiating a cascade reaction that leads to a storm of reactive oxygen species (ROS). Furthermore, Cu2O@Au nanozyme consumes glutathione and reduces the expression of the SLC7A11 (xCT) protein to decrease cancer cell uptake of cysteine, further enhancing the burst of ROS, resulting in lipid peroxidation in tumor cells and ultimately leading to ferroptosis. The excellent photothermal performance of Cu2O@Au can further enhance CDT. Additionally, Cu2O@Au nanozyme also has computed tomography (CT) and photothermal imaging capabilities. In conclusion, Cu2O@Au nanozymes, acting as tumor energy homeostasis disruptor, can effectively inhibit tumor growth and successfully achieve the synergistic effects of starvation therapy/CDT/photothermal therapy (PTT). This multifunctional nanozyme holds promise for providing valuable insights and therapeutic strategies for cancer treatment.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


