A portable, rapid isothermal amplification kit enabling naked eye detection of SARS-CoV-2 RNAs
IF 6.1
1区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Since the coronavirus disease 2019 (COVID-19) pandemic, isothermal amplification techniques have attracted attention due to their higher sensitivity and specificity, compared with immunoassays, and their potential application for point-of-care testing (POCT). A requirement of isothermal amplification-based POCT kits is the inclusion of a heating source with an electrical power supply. We developed an amplification-based rapid kit, which is a portable and naked eye-detectable reverse transcriptase (RT)-recombinase polymerase amplification (RPA) kit. The rapid RT-RPA kit consists of a flow-controllable paper chip, a nickel-chromium (NiCr)-based Joule-heating thin film, and a small-sized portable battery. We found that the Joule-heating thin film, powered by a lithium-ion battery (7.5 g, 20 mm × 35 mm size), was able to maintain the required temperature for the RPA reaction. After the RPA reaction, which takes approximately 20 min, the flow-controllable paper chip automatically enabled visualization of the amplicon by time-delayed release of gold nanoparticle-based optical probes. Using this system, we successfully detected severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at levels as low as 10 copies μL−1, within 30 min.
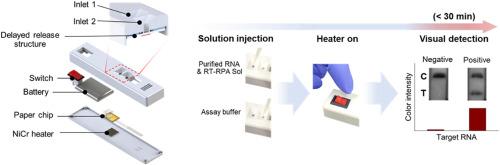
一种便携式、快速等温扩增试剂盒,可用于肉眼检测SARS-CoV-2 rna。
自2019冠状病毒病(COVID-19)大流行以来,与免疫分析法相比,等温扩增技术因其更高的灵敏度和特异性以及在护理点检测(POCT)中的潜在应用而受到关注。基于等温扩增的POCT试剂盒的要求是包含一个带电源的热源。我们开发了一种基于扩增的快速试剂盒,这是一种便携式裸眼检测逆转录酶(RT)-重组酶聚合酶扩增(RPA)试剂盒。快速RT-RPA套件由一个流动可控的纸芯片、一个基于镍铬(NiCr)的焦耳加热薄膜和一个小型便携式电池组成。我们发现,由锂离子电池(7.5 g, 20 mm × 35 mm尺寸)供电的焦耳加热薄膜能够维持RPA反应所需的温度。经过大约20分钟的RPA反应后,流动可控的纸芯片通过延时释放金纳米粒子光学探针自动实现扩增子的可视化。利用该系统,我们成功地在30分钟内检测出低至10拷贝μL-1的严重急性呼吸综合征冠状病毒2 (SARS-CoV-2)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Talanta
化学-分析化学
CiteScore
12.30
自引率
4.90%
发文量
861
审稿时长
29 days
期刊介绍:
Talanta provides a forum for the publication of original research papers, short communications, and critical reviews in all branches of pure and applied analytical chemistry. Papers are evaluated based on established guidelines, including the fundamental nature of the study, scientific novelty, substantial improvement or advantage over existing technology or methods, and demonstrated analytical applicability. Original research papers on fundamental studies, and on novel sensor and instrumentation developments, are encouraged. Novel or improved applications in areas such as clinical and biological chemistry, environmental analysis, geochemistry, materials science and engineering, and analytical platforms for omics development are welcome.
Analytical performance of methods should be determined, including interference and matrix effects, and methods should be validated by comparison with a standard method, or analysis of a certified reference material. Simple spiking recoveries may not be sufficient. The developed method should especially comprise information on selectivity, sensitivity, detection limits, accuracy, and reliability. However, applying official validation or robustness studies to a routine method or technique does not necessarily constitute novelty. Proper statistical treatment of the data should be provided. Relevant literature should be cited, including related publications by the authors, and authors should discuss how their proposed methodology compares with previously reported methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


