General chemoselective hindered amide coupling enabled by TCFH-catalytic Oxyma and transient imine protection†
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
We report a general chemoselective strategy for amide bond formation with poorly nucleophilic amines in the presence of reactive primary alcohols or amines as the competing nucleophiles. The selectivity for less reactive amines over competing alcohols was achieved using TCFH and catalytic Oxyma as a highly reactive, inexpensive, and safe reagent combination. By temporarily masking more reactive amines as imines through the use of electron-deficient aldehydes, the hindered amines could be similarly coupled with high efficiency and selectivity.
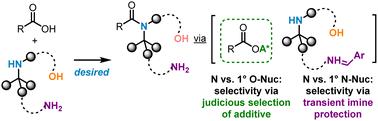
通过tcfh催化氧和瞬态亚胺保护实现的一般化学选择性阻碍酰胺偶联。
我们报道了在反应性伯醇或胺作为竞争性亲核试剂存在的情况下,与亲核性差的胺形成酰胺键的一般化学选择策略。使用TCFH和催化氧作为高活性、廉价和安全的试剂组合,实现了对反应性较低的胺相对于竞争醇的选择性。通过使用缺电子醛暂时掩盖更多的反应性胺作为亚胺,受阻胺可以类似地与高效率和选择性相结合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


