Bacterial Metallostasis: Metal Sensing, Metalloproteome Remodeling, and Metal Trafficking
IF 51.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Transition metals function as structural and catalytic cofactors for a large diversity of proteins and enzymes that collectively comprise the metalloproteome. Metallostasis considers all cellular processes, notably metal sensing, metalloproteome remodeling, and trafficking (or allocation) of metals that collectively ensure the functional integrity and adaptability of the metalloproteome. Bacteria employ both protein and RNA-based mechanisms that sense intracellular transition metal bioavailability and orchestrate systems-level outputs that maintain metallostasis. In this review, we contextualize metallostasis by briefly discussing the metalloproteome and specialized roles that metals play in biology. We then offer a comprehensive perspective on the diversity of metalloregulatory proteins and metal-sensing riboswitches, defining general principles within each sensor superfamily that capture how specificity is encoded in the sequence, and how selectivity can be leveraged in downstream synthetic biology and biotechnology applications. This is followed by a discussion of recent work that highlights selected metalloregulatory outputs, including metalloproteome remodeling and metal allocation by metallochaperones to both client proteins and compartments. We close by briefly discussing places where more work is needed to fill in gaps in our understanding of metallostasis.
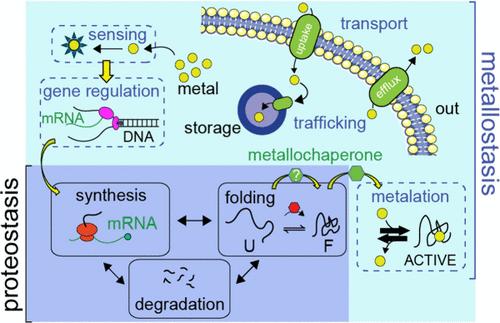
细菌金属滞留:金属传感、金属蛋白质组重塑和金属运输
过渡金属是组成金属蛋白组的多种蛋白质和酶的结构辅助因子和催化辅助因子。金属稳态考虑所有的细胞过程,特别是金属传感、金属蛋白质组重塑和金属的运输(或分配),这些过程共同确保金属蛋白质组的功能完整性和适应性。细菌利用基于蛋白质和rna的机制来感知细胞内过渡金属的生物利用度,并协调系统水平的输出来维持金属平衡。在这篇综述中,我们通过简要讨论金属蛋白质组和金属在生物学中发挥的特殊作用来背景金属停滞。然后,我们提供了金属调节蛋白和金属传感核糖开关多样性的全面视角,定义了每个传感器超家族的一般原理,这些超家族捕获了如何在序列中编码特异性,以及如何在下游合成生物学和生物技术应用中利用选择性。随后讨论了最近的工作,重点介绍了金属调节输出,包括金属蛋白组重塑和金属伴侣蛋白对客户蛋白和区室的金属分配。最后,我们简要地讨论了需要做更多工作来填补我们对金属平衡理解的空白的地方。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Reviews
化学-化学综合
CiteScore
106.00
自引率
1.10%
发文量
278
审稿时长
4.3 months
期刊介绍:
Chemical Reviews is a highly regarded and highest-ranked journal covering the general topic of chemistry. Its mission is to provide comprehensive, authoritative, critical, and readable reviews of important recent research in organic, inorganic, physical, analytical, theoretical, and biological chemistry.
Since 1985, Chemical Reviews has also published periodic thematic issues that focus on a single theme or direction of emerging research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


