A Magnetically Actuated MOF-Based Nanozyme for Intensified Induction of Ferroptosis and Immunogenic Cell Death Via Autophagy Blockade
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
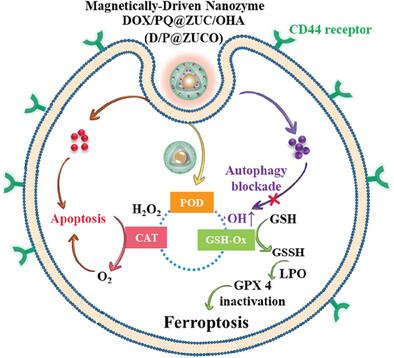
Nanozymes mimicking enzymes show great promise in anti-tumor therapy but are often limited by their low catalytic activity and lack of tumor specificity in hostile tumor microenvironments. This study develops a novel nanozyme, D/P@ZUCO, utilizing metal–organic frameworks (MOFs) with glutathione oxidase, peroxidase, and catalase-like activities. D/P@ZUCO is synthesized using ZnFe2O4 and NH2-UiO66 (Cu/Zr) through an in situ growth method, followed by loading with doxorubicin (DOX) and primaquine (PQ), and functionalization with oxidized hyaluronic acid (OHA). It efficiently catalyzes the conversion of hydrogen peroxide (H2O2) into hydroxyl radicals (·OH) and glutathione (GSH) into glutathione disulfide (GSSH), initiating ferroptosis in cancer cells. Additionally, the conversion of excess H2O2 into oxygen (O2) enhances the apoptosis effects of DOX. Importantly, the inhibition of autophagy by D/P@ZUCO exacerbates ferroptosis and immunogenic cell death (ICD), triggering a potent anti-tumor immune response. The targeted drug delivery of D/P@ZUCO is facilitated by magnetic guidance and interactions between OHA and CD44 receptors. D/P@ZUCO demonstrates effective cancer treatment by triggering multiple cell death pathways through a synergistic combination of enzymatic actions, serving as a paradigm for systemic activation of multiple enzymes in triple-negative breast cancer therapy.
磁驱动mof纳米酶通过自噬阻断强化诱导铁凋亡和免疫原性细胞死亡
纳米酶模拟酶在抗肿瘤治疗中显示出巨大的前景,但往往受到其低催化活性和缺乏肿瘤特异性在敌对肿瘤微环境中的限制。本研究开发了一种新型纳米酶D/P@ZUCO,利用具有谷胱甘肽氧化酶、过氧化物酶和过氧化氢酶样活性的金属有机框架(mof)。以ZnFe2O4和NH2-UiO66 (Cu/Zr)为原料,通过原位生长法合成D/P@ZUCO,然后用阿霉素(DOX)和伯氨喹(PQ)负载,氧化透明质酸(OHA)功能化。它能有效地催化过氧化氢(H2O2)转化为羟基自由基(·OH)和谷胱甘肽(GSH)转化为谷胱甘肽二硫醚(GSSH),引发癌细胞铁死亡。此外,过量H2O2转化为氧气(O2)增强了DOX的凋亡作用。重要的是,D/P@ZUCO对自噬的抑制加剧了铁凋亡和免疫原性细胞死亡(ICD),引发了有效的抗肿瘤免疫反应。D/P@ZUCO的靶向药物递送是通过磁引导和OHA和CD44受体之间的相互作用来促进的。D/P@ZUCO通过酶作用的协同组合触发多种细胞死亡途径,证明了有效的癌症治疗,可作为三阴性乳腺癌治疗中多种酶的全身激活的范例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
文献相关原料
公司名称
产品信息
麦克林
Acetic acid
麦克林
Copper chloride dihydrate (CuCl2·2H2O)
麦克林
Anhydrous sodium acetate (NaAc)
阿拉丁
3-mercaptopropionic acid (3-MPA)
阿拉丁
Sodium periodate (NaIO4)
阿拉丁
Zinc chloride (ZnCl2)
阿拉丁
Ferric chloride hexahydrate (FeCl3·6H2O)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


