Trapping Properties of Iodine, Cesium, and Tellurium in Uranium Dioxide: A DFT+U Study
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
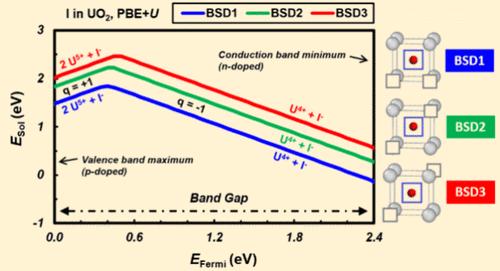
We investigate the trapping properties of iodine, cesium, and tellurium in uranium dioxide, using the Hubbard-corrected density functional theory (DFT+U). In order to avoid the metastable states inherent to this method, we use the occupation matrix control (OMC) scheme, which also allows us to monitor the oxidation states of the different species. The most favorable trapping sites, oxidation states, and solubility of I, Cs, and Te are evaluated in stoichiometric UO2. To that end, vacancy-like defects under various charge states, including uranium and oxygen vacancies, U–O divacancy and bound Schottky defects, as well as the interstitial position, are considered as potential trapping sites in UO2. Te is found to exhibit a wide range of possible oxidation states, ranging from Te– to Te4+, depending on the stable trapping site considered. For I and Cs, one predominant oxidation state for each fission product, namely, I– and Cs+, is found. This behavior is mainly accommodated by the charge of the defects. By providing accurate trapping sites and oxidation states of volatile fission products in UO2, this study is expected to contribute in the development of larger scale simulation methods, enabling a better prediction and mitigation of corrosion issues in nuclear fuel cladding.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


