Redox Active vs Redox Neutral in Ru/Pd-Catalyzed Sulfonylation: Theoretical Insights into Structure–Activity Relationship between Metal Centers and Regio-Selectivity
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The structure–activity relationship between the metal center and regio-selectivity is persistently a pivotal scientific issue. To address this, we select the 2-phenylpyridine sulfonylation reactions catalyzed by ruthenium and palladium as research subjects. An extensive theoretical study has been conducted on their reaction mechanisms, the sources of regio-selectivity, and the evolution of electronic structures. The distinct electronic structures lead to completely different catalytic mechanisms and electronic structure evolution processes for ruthenium and palladium. Ruthenium tends to form six-coordinate octahedral complexes, thus undergoing an inner-sphere redox active Ru(II)–Ru(III)–Ru(IV)–Ru(II) catalytic cycle. In contrast, palladium tends to form four-coordinate planar quadrilateral complexes, hence undergoing an outer-sphere redox neutral Pd(II) catalytic cycle. The distinct electronic structure evolution processes fundamentally differentiate the radical attack modes in the sulfonation process, thereby determining the regio-selectivity of the reaction. In the Ru-catalyzed system, the meta-selectivity arises mainly from a more stable Schrock carbene-type meta-intermediate. For the Pd-catalyzed system, the ortho-selectivity mainly comes from the stabilizing effect of the Pd(II) center on the single electron. This study provides novel insights into how the electronic structure of metal centers influences the reaction mechanism and selectivity, making a theoretical contribution to a deeper comprehension of the mechanism and regio-selectivity underlying aromatic functionalization reactions.
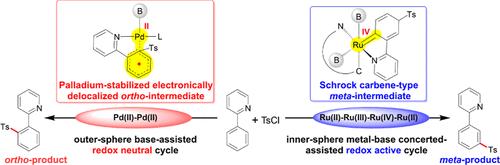
Ru/ pd催化磺化反应中的氧化还原活性与氧化还原中性:金属中心与区域选择性构效关系的理论见解
金属中心与区域选择性之间的构效关系一直是一个关键的科学问题。为了解决这一问题,我们选择钌和钯催化的2-苯基吡啶磺化反应作为研究对象。对它们的反应机理、区域选择性的来源和电子结构的演化进行了广泛的理论研究。不同的电子结构导致钌和钯的催化机理和电子结构演化过程完全不同。钌倾向于形成六配位八面体配合物,从而发生球内氧化还原活性Ru(II) -Ru (III) -Ru (IV) -Ru (II)催化循环。相反,钯倾向于形成四坐标平面四边形配合物,因此经历了一个球外氧化还原中性Pd(II)催化循环。不同的电子结构演化过程从根本上区分了磺化过程中自由基的攻击方式,从而决定了反应的区域选择性。在钌催化体系中,元选择性主要来自于更稳定的施罗克卡宾型中间产物。对于Pd催化体系,邻选择性主要来自于Pd(II)中心对单电子的稳定作用。本研究对金属中心的电子结构如何影响反应机理和选择性提供了新的见解,为深入理解芳香官能化反应的机理和区域选择性做出了理论贡献。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


