Enhanced Nano-LC-MS for Analyzing Dansylated Oral Cancer Tissue Metabolome Dissolved in Solvents with High Elution Strength
IF 5.7
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Background
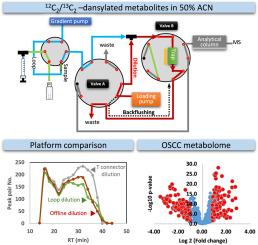
Tissue metabolomics analysis, alongside genomics and proteomics, offers crucial insights into the regulatory mechanisms of tumorigenesis. To enhance metabolite detection sensitivity, chemical isotope labeling (CIL) techniques, such as dansylation, have been developed to improve metabolite separation and ionization in mass spectrometry (MS). However, the dissolution of hydrophobic derivatized metabolites in solvents with high acetonitrile content limits the use of liquid chromatography (LC) systems with small-volume reversed-phase (RP) columns. In this study, we established a nano-LC-MS system with an online dilution design to address this issue, enabling sensitive analysis of oral cancer tissue metabolomes.Results
Our nano-LC system features a flow path design with online sample dilution before an RP trap column and backflushing of the trap column before entering the analytical column. Compared to other nano-LC systems, both with and without online dilution designs, our system demonstrates the superiority of the T-connector-based dilution method. Using only 1/20th of the sample required for popular micro-LC systems, our nano-LC detects a larger number of peak pairs with similar recovery rates for both hydrophilic and hydrophobic metabolites, ensuring unbiased results. Thirty-two matched pairs of oral squamous cell carcinoma (OSCC) tissue samples and adjacent noncancerous tissues (ANTs) underwent high-throughput CIL-metabolome analysis using our nano-LC system. Compared to our previous micro-LC methods, the nano-LC-MS system exhibits enhanced detection sensitivity, significantly reducing sample requirements.Significance
Our findings highlight the efficacy of our platform for metabolomic analysis with limited sample amounts. The nano-LC system’s ability to analyze samples dissolved in strong eluents suggests potential applications for handling other hydrophobic compounds using RPLC or other separation methods facing similar solvent incompatibility issues. This approach holds promise for identifying novel metabolite biomarkers for oral cancers, advancing our understanding of tumorigenesis, and enhancing clinical applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


