Construction of Mo2TiC2Tx MXene as a Superior Carrier to Support Ru–CuO Heterojunctions for Improving Alkaline Hydrogen Oxidation
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
The sluggish anodic hydrogen oxidation reaction (HOR) of the hydroxide exchange membrane fuel cell (HEMFC) is a significant barrier for practical implementation. Herein, we designed a catalyst of Mo2TiC2Tx MXene-supported Ru–CuO heterojunctions (named as Ru–CuO/MXene). The XPS spectra and the d-band center data of the different amounts of Cu of the Ru–CuO/MXene suggested that there existed a strongly electronic metal–support interaction between the active species and the substrate with MXene as the excellent carrier. Furthermore, the in situ electrochemical experimental results (operando electrochemical impedance spectroscopy and in situ electrochemical Raman spectroscopy) and density functional theory (DFT) calculations showed that Ru and CuO were the optimal adsorption sites for surface species *H and *OH, respectively, endowing Ru–CuO/MXene with the ability to weaken the hydrogen binding energy (HBE) and strengthen the hydroxide binding energy (OHBE). Remarkably, the optimized catalyst modified an impressive HOR activity in the 0.1 M KOH electrolyte with a kinetic and an exchange current density of 7.63 and 1.37 mA cm–2 at 50 mV overpotential (vs. RHE), respectively, which were 1.98- and 1.2-fold higher than those of commercial Pt/C (20 wt %). Finally, the as-prepared catalyst also exhibited superior durability and exceptional CO antipoisoning ability in 1000 ppm of the CO/H2-saturated 0.1 M KOH electrolyte. This work provides an inspiring strategy to design high-activity HOR electrocatalyst-based metallic Ru in alkaline environments.
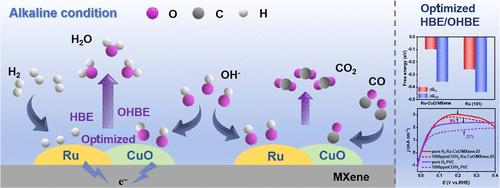
Mo2TiC2Tx MXene作为支持Ru-CuO异质结改善碱性氢氧化的优良载体的构建
氢氧交换膜燃料电池(HEMFC)的阳极氢氧化反应(HOR)缓慢是其实际应用的一个重要障碍。为此,我们设计了一种Mo2TiC2Tx MXene负载的Ru-CuO异质结催化剂(命名为Ru-CuO /MXene)。不同Cu含量的Ru-CuO /MXene的XPS光谱和d波段中心数据表明,活性物质与基体之间存在强烈的电子-金属支撑相互作用,MXene为优异的载体。此外,原位电化学实验结果(operando电化学阻抗谱和原位电化学拉曼光谱)和密度泛函理论(DFT)计算表明,Ru和CuO分别是表面物质*H和*OH的最佳吸附位点,使Ru - CuO/MXene具有削弱氢结合能(HBE)和增强氢氧结合能(OHBE)的能力。值得注意的是,优化后的催化剂在0.1 M KOH电解液中具有令人惊叹的HOR活性,在50 mV过电位(相对于RHE)下,其动力学和交换电流密度分别为7.63和1.37 mA cm-2,比商用Pt/C (20 wt %)高1.98和1.2倍。最后,所制备的催化剂在1000ppm的CO/ h2饱和0.1 M KOH电解质中也表现出优异的耐久性和优异的CO抗中毒能力。这项工作为在碱性环境中设计高活性的基于HOR电催化剂的金属Ru提供了一个鼓舞人心的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


