Boosting the Performance of Electrochemical CO2 Reduction to HCOOH through the Interaction of Pyridinic Nitrogen with Sn
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The development of efficient and stable catalysts for electrochemical CO2 reduction (CO2RR) to formic acid (HCOOH) is of great practical significance for balancing energy and environmental issues. SnO2 shows potential application of the CO2RR to HCOOH, while its low current carrier density and inappropriate adsorption energy for crucial intermediates limit its performance in terms of activity and selectivity. In this study, phthalocyanine (Pc) and tetraphenyl porphyrin (TPP) were loaded onto SnO2 nanosheets forming composite materials with different types of N (SnO2/Pc and SnO2/TPP), respectively. In Pc, both pyridinic N and pyrrolic N are present, while only pyrrolic N is present for TPP. XPS analysis reveals that the obvious electronic interaction happened between the pyridinic N and Sn, regulating the electronic states of Sn sites. As a result, SnO2/Pc composites can selectively convert CO2 to HCOOH with a Faraday efficiency up to 90.25% and partial current density up to 16.15 mA cm–2 at −1.3 V vs RHE, higher than SnO2/TPP and SnO2. Density functional theory (DFT) calculations further prove that the superior catalytic performance of SnO2/Pc comes from its moderate adsorption energy for *OCHO and *HCOOH, which is beneficial for *HCOOH desorption.
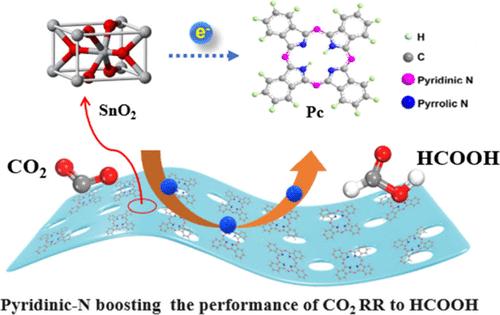
吡啶氮与Sn相互作用提高CO2电化学还原成HCOOH的性能
开发高效稳定的电化学还原CO2 (CO2RR)制甲酸(HCOOH)催化剂,对于平衡能源与环境问题具有重要的现实意义。SnO2显示了CO2RR在HCOOH上的潜在应用,但其电流载流子密度低,对关键中间体的吸附能量不合适,限制了其活性和选择性的性能。在本研究中,将酞菁(Pc)和四苯基卟啉(TPP)分别负载在SnO2纳米片上,形成不同类型N (SnO2/Pc和SnO2/TPP)的复合材料。Pc中同时存在吡啶N和吡啶N,而TPP中只存在吡啶N。XPS分析表明,吡啶N与Sn之间发生了明显的电子相互作用,调节了Sn位的电子态。结果表明,SnO2/Pc复合材料在−1.3 V vs RHE下可选择性地将CO2转化为HCOOH,法拉第效率高达90.25%,分电流密度高达16.15 mA cm-2,高于SnO2/TPP和SnO2。密度泛函理论(DFT)计算进一步证明了SnO2/Pc优越的催化性能来源于其对*OCHO和*HCOOH的中等吸附能,有利于*HCOOH的脱附。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


