How NINJ1 mediates plasma membrane rupture and why NINJ2 cannot
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Ninjurin-1 (NINJ1) is an active executioner of plasma membrane rupture (PMR), a process previously thought to be a passive osmotic lysis event in lytic cell death. Ninjurin-2 (NINJ2) is a close paralog of NINJ1 but cannot mediate PMR. Using cryogenic electron microscopy (cryo-EM), we show that NINJ1 and NINJ2 both assemble into linear filaments that are hydrophobic on one side but hydrophilic on the other. This structural feature and other evidence point to a PMR mechanism by which NINJ1 filaments wrap around and solubilize membrane fragments and, less frequently, form pores in the plasma membrane. In contrast to the straight NINJ1 filament, the NINJ2 filament is curved toward the intracellular space, preventing its circularization or even assembly on a relatively flat membrane to mediate PMR. Mutagenesis studies further demonstrate that the NINJ2 filament curvature is induced by strong association with lipids, particularly a cholesterol molecule, at the cytoplasmic leaflet of the lipid bilayer.
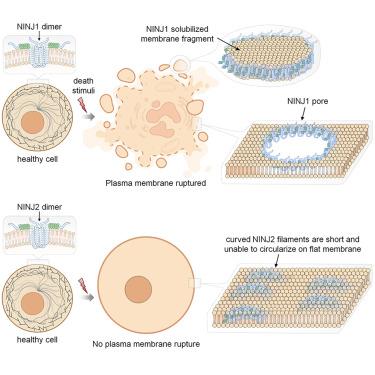
NINJ1如何介导质膜破裂,为什么NINJ2不能
Ninjurin-1 (Ninjurin-1)是质膜破裂(PMR)的主动刽子手,这一过程以前被认为是溶解性细胞死亡中的被动渗透裂解事件。Ninjurin-2 (NINJ2)是NINJ1的近亲,但不能调解PMR。利用低温电子显微镜(cryo-EM),我们发现NINJ1和NINJ2都组装成一边疏水而另一边亲水的线状细丝。这一结构特征和其他证据表明,通过PMR机制,NINJ1细丝缠绕并溶解膜碎片,并且在质膜上形成孔,这种情况较少。与直的NINJ1丝相反,NINJ2丝向细胞内空间弯曲,阻止其圆化甚至组装在相对平坦的膜上来介导PMR。诱变研究进一步表明,NINJ2纤维弯曲是由脂质双分子层细胞质小叶上的脂质,特别是胆固醇分子的强关联诱导的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


