Finerenone, Obesity, and Heart Failure With Mildly Reduced/Preserved Ejection Fraction
IF 22.3
1区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background
Obesity is associated with excessive adipocyte-derived aldosterone secretion, independent of the classical renin-angiotensin-aldosterone cascade, and mineralocorticoid receptor antagonists may be more effective in patients with heart failure (HF) and obesity.
Objectives
This study sought to examine the effects of the nonsteroidal mineralocorticoid receptor antagonist finerenone compared with placebo, according to body mass index (BMI) in FINEARTS-HF (FINerenone trial to investigate Efficacy and sAfety superioR to placebo in paTientS with Heart Failure).
Methods
A total of 6,001 patients with HF with NYHA functional class II, III, and IV, a left ventricular ejection fraction of ≥40%, evidence of structural heart disease, and elevated natriuretic peptide levels were randomized to finerenone or placebo. BMI (kg/m2) was examined using World Health Organization categories, namely, underweight/normal weight (<25.0 kg/m2; n = 1,306); overweight (25.0-29.9 kg/m2; n = 1,990); obesity class I (30.0-34.9 kg/m2; n = 1,546); obesity class II (35.0-39.9 kg/m2; n = 751); and obesity class III (≥40 kg/m2; n = 395). The primary outcome was cardiovascular death and total worsening HF events.
Results
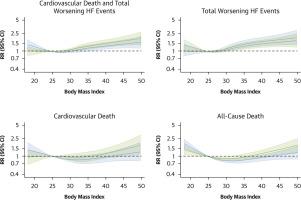
Data on baseline BMI were available for 5,988 patients (median: 29.2 kg/m2; Q1-Q3: 25.5-33.6 kg/m2). Compared with patients who were underweight/normal weight, those with obesity class II or III had a higher risk of the primary outcome (underweight/normal weight, reference; overweight, unadjusted rate ratio: 0.96 [95% CI: 0.81-1.15]; obesity class I: 1.04 [95% CI: 0.86-1.26]; obesity class II-III: 1.26 [95% CI: 1.03-1.54]). The effect of finerenone on the primary outcome did not vary by baseline BMI (underweight/normal weight, rate ratio: 0.80 [95% CI: 0.62-1.04]; overweight: 0.91 [95% CI: 0.72-1.15]; obesity class I: 0.92 [95% CI: 0.72-1.19]; obesity class II-III: 0.67 [95% CI: 0.50-0.89]; Pinteraction = 0.32). However, when BMI was examined as a continuous variable, the beneficial effect of finerenone seemed to be greater in those with a higher BMI (Pinteraction = 0.005). A similar pattern was observed for total worsening HF events. Consistent effects across baseline BMI were observed for cardiovascular and all-cause death and improvement in the Kansas City Cardiomyopathy Questionnaire scores.
Conclusions
In patients with HF with mildly reduced/preserved ejection fraction, the beneficial effects of finerenone on clinical events and symptoms were consistent, irrespective of BMI at baseline, possibly with a greater effect on the primary outcome in patients with higher BMI. (FINEARTS-HF [FINerenone trial to investigate Efficacy and sAfety superioR to placebo in paTientS with Heart Failure]; NCT04435626)
芬芬烯酮、肥胖和心力衰竭伴射血分数轻度降低/保留:fineards - hf的预先分析
背景:肥胖与脂肪细胞衍生的醛固酮分泌过多有关,独立于经典的肾素-血管紧张素-醛固酮级联反应,矿皮质激素受体拮抗剂可能对心力衰竭(HF)和肥胖患者更有效。目的:本研究旨在根据finhearts - hf (finerenone试验研究心力衰竭患者finerenone优于安慰剂的疗效和安全性)的体重指数(BMI),研究非甾体类矿物皮质激素受体拮抗剂芬尼酮与安慰剂的效果。方法共6001例心衰患者,NYHA功能等级为II、III和IV级,左心室射血分数≥40%,有结构性心脏病证据,利钠肽水平升高,随机分为芬尼酮组和安慰剂组。BMI (kg/m2)采用世界卫生组织分类进行检查,即体重不足/正常体重(25.0 kg/m2;n = 1,306);超重(25.0-29.9 kg/m2;n = 1990);肥胖I级(30.0-34.9 kg/m2);n = 1546);肥胖II级(35.0 ~ 39.9 kg/m2);n = 751);III类肥胖(≥40 kg/m2);n = 395)。主要终点是心血管死亡和总心衰恶化事件。结果5988例患者获得了基线BMI数据(中位数:29.2 kg/m2;Q1-Q3: 25.5-33.6 kg/m2)。与体重不足/正常体重的患者相比,II级或III级肥胖患者的主要结局(体重不足/正常体重,参考;超重,未调整比率:0.96 [95% CI: 0.81-1.15];肥胖I类:1.04 [95% CI: 0.86-1.26];II-III级肥胖:1.26 [95% CI: 1.03-1.54])。芬尼酮对主要结局的影响没有因基线BMI而变化(体重不足/正常体重,比率比:0.80 [95% CI: 0.62-1.04];超重:0.91 [95% CI: 0.72-1.15];肥胖I类:0.92 [95% CI: 0.72-1.19];II-III级肥胖:0.67 [95% CI: 0.50-0.89];p交互作用= 0.32)。然而,当BMI作为一个连续变量进行检查时,细芬烯酮的有益效果似乎在BMI较高的人群中更大(p交互作用= 0.005)。在心力衰竭总恶化事件中也观察到类似的模式。观察到基线BMI对心血管和全因死亡的一致影响,以及堪萨斯城心肌病问卷评分的改善。结论:在射血分数轻度降低/保留的HF患者中,芬尼酮对临床事件和症状的有益作用是一致的,与基线时的BMI无关,可能对高BMI患者的主要结局有更大的影响。finhearts - hf [FINerenone在心力衰竭患者中的疗效和安全性优于安慰剂的试验];NCT04435626)
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
42.70
自引率
3.30%
发文量
5097
审稿时长
2-4 weeks
期刊介绍:
The Journal of the American College of Cardiology (JACC) publishes peer-reviewed articles highlighting all aspects of cardiovascular disease, including original clinical studies, experimental investigations with clear clinical relevance, state-of-the-art papers and viewpoints.
Content Profile:
-Original Investigations
-JACC State-of-the-Art Reviews
-JACC Review Topics of the Week
-Guidelines & Clinical Documents
-JACC Guideline Comparisons
-JACC Scientific Expert Panels
-Cardiovascular Medicine & Society
-Editorial Comments (accompanying every Original Investigation)
-Research Letters
-Fellows-in-Training/Early Career Professional Pages
-Editor’s Pages from the Editor-in-Chief or other invited thought leaders

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


