Efficacy of Midostaurin Combined With Intensive Chemotherapy in Core Binding Factor Leukemia: A Phase II Clinical Trial
IF 10.1
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
Samples from 34 adult patients newly diagnosed with core binding factor leukemia (CBFL) were collected both at the time of diagnosis and at relapse and were centrally analyzed. Eligible patients received either standard induction CT known as “3 + 7” or an equivalent regimen, according to the recruiting center's policy. Patients who achieved CR or CRi received 3 courses of high-dose ARA-C (Cytarabine) 3000 mg/m2 every 12 h on days 1, 3, and 5, along with midostaurin at the dose of 50 mg b.i.d from Day 8 to Day 21 as part of consolidation therapy. Following the completion of the consolidation phase, patients received midostaurin as a monotherapy at the dose of 50 mg b.i.d. for 1 year as continuation therapy. The CR rate was 97%; we recorded an OS rate of 73.52% and a DFS rate of 48.4% for the entire cohort. The RI was 38.8% in the CBFB::MYH11 and 66.6% in the RUNX1::RUNX1T1 group. MRD (Measurable Residual Disease) was assessed by RQ-PCR at 10 time points throughout the study, as indicated by arrows.
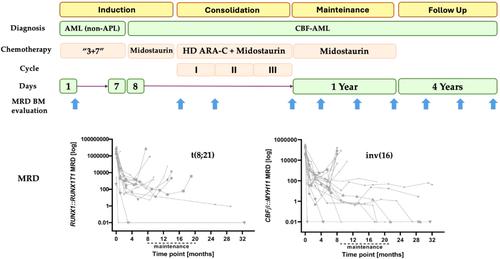
该研究收集了34名新确诊的核心结合因子白血病(CBFL)成人患者在确诊时和复发时的样本,并进行了集中分析。符合条件的患者根据招募中心的政策接受了被称为 "3 + 7 "的标准诱导 CT 或同等方案。达到CR或CRi的患者接受3个疗程的大剂量ARA-C(阿糖胞苷)治疗,剂量为3000 mg/m2,每12小时一次,疗程为第1、3和5天,同时从第8天到第21天使用剂量为50 mg b.i.d的米哚妥林作为巩固治疗的一部分。巩固治疗阶段结束后,患者继续接受米哚妥林单药治疗,剂量为50毫克/天,疗程为1年。CR率为97%;我们的记录显示,整个队列的OS率为73.52%,DFS率为48.4%。CBFB::MYH11组的RI为38.8%,RUNX1::RUNX1T1组的RI为66.6%。如箭头所示,在整个研究的 10 个时间点通过 RQ-PCR 评估 MRD(可测量残留病灶)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
15.70
自引率
3.90%
发文量
363
审稿时长
3-6 weeks
期刊介绍:
The American Journal of Hematology offers extensive coverage of experimental and clinical aspects of blood diseases in humans and animal models. The journal publishes original contributions in both non-malignant and malignant hematological diseases, encompassing clinical and basic studies in areas such as hemostasis, thrombosis, immunology, blood banking, and stem cell biology. Clinical translational reports highlighting innovative therapeutic approaches for the diagnosis and treatment of hematological diseases are actively encouraged.The American Journal of Hematology features regular original laboratory and clinical research articles, brief research reports, critical reviews, images in hematology, as well as letters and correspondence.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


