Low Potential Electrochemical CO2 Reduction to Methanol over Nickel-Based Hollow 0D Carbon Superstructure
IF 24.4
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
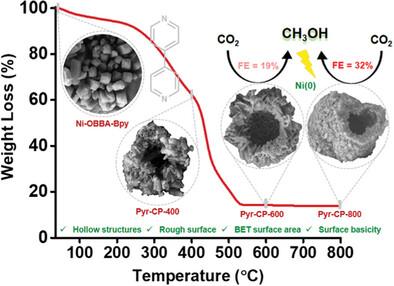
Electrochemical carbon dioxide reduction reaction (CO2RR) to valuable fuels and chemical feedstock is a sustainable strategy to lower the anthropogenic CO2 concentration, thereby dynamising the carbon cycle in the environment. CH3OH on the other hand is undoubtedly the most desirable C1 product of CO2RR. However, selective electroreduction of CO2-to-CH3OH is very challenging and only limited catalysts are reported in literature. Pyrolyzing metal-organic frameworks (MOFs) to generate carbon matrix impregnated with metal nanoparticles, heralds exciting electrocatalytic properties. This study unveiled the morphological evolution of a mixed-ligand Ni-MOF (Ni-OBBA-Bpy) during pyrolysis, to generate Ni nanoparticles anchored 0D porous hollow carbon superstructures (Pyr-CP-800 and Pyr-CP-600). This unique morphology invokes high specific surface area and surface roughness to the materials, which synergistically facilitates the selective electroreduction of CO2-to-CH3OH. In comparison to most of the previously reported Ni electrocatalysts that mainly produced CO, Pyr-CP-800 selectively yielded CH3OH with Faradaic efficiency (FE) of 32.46% at −0.60 V versus RHE (reversible hydrogen electrode) in 1.0 M KOH solution, which is highest among other reported Ni-based electrocatalysts in the literature, to best of our knowledge. Additionally, insights from density functional theory (DFT) calculations revealed that Ni (111) plane to be the active site toward the electrochemical. CO2-to-CH3OH formation.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Energy Materials
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
41.90
自引率
4.00%
发文量
889
审稿时长
1.4 months
期刊介绍:
Established in 2011, Advanced Energy Materials is an international, interdisciplinary, English-language journal that focuses on materials used in energy harvesting, conversion, and storage. It is regarded as a top-quality journal alongside Advanced Materials, Advanced Functional Materials, and Small.
With a 2022 Impact Factor of 27.8, Advanced Energy Materials is considered a prime source for the best energy-related research. The journal covers a wide range of topics in energy-related research, including organic and inorganic photovoltaics, batteries and supercapacitors, fuel cells, hydrogen generation and storage, thermoelectrics, water splitting and photocatalysis, solar fuels and thermosolar power, magnetocalorics, and piezoelectronics.
The readership of Advanced Energy Materials includes materials scientists, chemists, physicists, and engineers in both academia and industry. The journal is indexed in various databases and collections, such as Advanced Technologies & Aerospace Database, FIZ Karlsruhe, INSPEC (IET), Science Citation Index Expanded, Technology Collection, and Web of Science, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


