Elucidating the Mechanism of Lithium Ion Extraction in a Tributyl Phosphate-Ionic Liquid Mixed System from the Perspective of Water Coordination
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
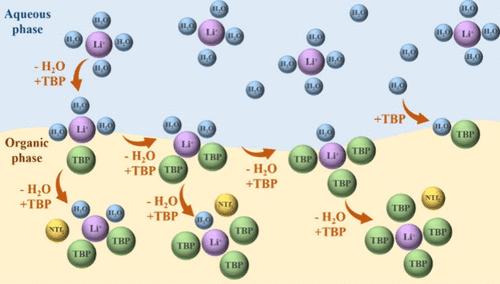
With the rapid development of the lithium-ion battery industry, the demand for lithium resources is becoming more and more urgent. Lithium extraction is a widely used process; especially, tributyl phosphate (TBP) systems have attracted much attention. During the extraction and purification process of lithium ions, the extractant TBP may encounter issues including aging, emulsification, precipitation, and low utilization rates. These problems arise due to an inadequate understanding of the extraction mechanism. In this work, the TBP-ionic liquid system was utilized to extract Li+, the variations of trace water in the organic phase were systematically and quantitatively investigated, and the extraction mechanism was further investigated. Experiments have confirmed that TBP forms a 1:1 complex with water. Various complex ratios of TBP, Li+, and water were determined, and the structures were verified by simulations. The complexes have three different ratios of TBP, Li+, and water: 2:1:2, 3:1:1, and 4:1:0. Water molecules are substituted during the complexation of Li+ with TBP, and meanwhile, the NTf2– anion equilibrates the charge of the Li+-TBP complex. This work provides experimental data and a theoretical basis for understanding Li+ extraction in the TBP-ionic liquid system, which is conducive to the sustainable development of the lithium industry.
从水配位的角度探讨磷酸三丁酯-离子液体混合体系中锂离子的萃取机理
随着锂离子电池产业的快速发展,对锂资源的需求越来越迫切。锂萃取是一种应用广泛的工艺;特别是磷酸三丁酯(TBP)体系引起了人们的广泛关注。在锂离子的提取纯化过程中,萃取剂TBP存在老化、乳化、沉淀、利用率低等问题。这些问题的产生是由于对萃取机理的认识不足。本文利用tbp离子液体体系萃取Li+,系统定量地考察了有机相中微量水的变化,并进一步探讨了萃取机理。实验证实TBP与水形成1:1的配合物。测定了TBP、Li+和水的不同复比,并通过模拟验证了其结构。这些配合物具有三种不同的TBP、Li+和水的比例:2:1:2、3:1:1和4:1:0。在Li+与TBP络合过程中,水分子被取代,同时,NTf2 -阴离子平衡Li+-TBP络合物的电荷。本工作为了解tbp离子液体体系中Li+的提取提供了实验数据和理论基础,有利于锂工业的可持续发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


