Self-Recycled electron donor resists disfavored oxidation reconstruction of Cu(I)-based electrocatalyst for nitrate removal by charge compensation
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
The overuse of nitrate has led to the accumulation in natural water, being a globe issue in environment and human health. Electrochemical NO3− reduction reaction (eNO3RR) to ammonia occurs under ambient condition with low energy consumption and the yield of value-added product, being promising for NO3− removal. Cu(I)-based eNO3RR catalysts suffer from unavoidable oxidation reconstruction to Cu(II), reducing the performance of NO3− removal. In this work, we demonstrate charge compensation strategy to resist oxidation reconstruction of Cu(I)-based eNO3RR catalysts by introducing self-recycled electron donor. Taking Ti(III)-modified Cu2O/Cu as the proof-of-concept model, electron donor Ti(III) can donate electron to Cu(II) to regenerate Cu(I), meanwhile the expended Ti(III) can be recycled from the generated Ti(IV) via intervalence charge transfer (IVCT). Benefiting from those, Ti-Cu2O/Cu-10 exhibits significantly improved activity and durability for NO3− removal compared to Cu2O/Cu. The percentage of NO3− removal keeps at ∼95.0 % with the initial concentration of 60 mg•L−1 NO3−-N at -0.9 V vs. RHE in 15 consecutive cycling tests (corresponding to 30 h). This work presents a feasible strategy to resist oxidation reconstruction of Cu(I)-based eNO3RR catalysts, making NO3− removal more effective, more durable, and more sustainable.

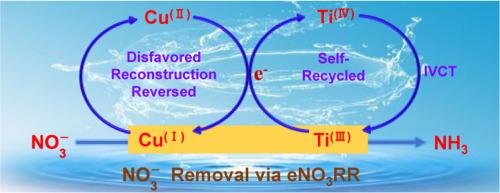
自回收电子供体电荷补偿法抗Cu(I)基电催化剂的不良氧化重构
硝酸盐的过度使用导致其在自然水体中积累,是一个全球性的环境和人类健康问题。在常温条件下,电化学还原NO3−反应(eNO3RR)生成氨,能耗低,产品附加值高,是脱除NO3−的好方法。Cu(I)基en3rr催化剂不可避免地会氧化重构为Cu(II),降低了去除NO3−的性能。在这项工作中,我们通过引入自回收电子供体,证明了电荷补偿策略来抵抗Cu(I)基en3rr催化剂的氧化重构。以Ti(III)修饰的Cu2O/Cu为概念验证模型,电子供体Ti(III)可以给电子给Cu(II)使Cu(I)再生,同时消耗的Ti(III)可以通过价间电荷转移(IVCT)从生成的Ti(IV)中回收。与Cu2O/Cu相比,Ti-Cu2O/Cu-10对NO3−的去除活性和持久性显著提高。在-0.9 V / RHE条件下,初始浓度为60 mg•L−1 NO3−-N,连续15次循环试验(对应30小时),NO3−去除率保持在~ 95.0%。本研究提出了一种可行的策略来抵抗Cu(I)基en3rr催化剂的氧化重建,使NO3−的去除更有效,更持久,更可持续。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


