Maternal gut microbiota influence stem cell function in offspring
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
The maternal microbiome influences child health. However, its impact on a given offspring’s stem cells, which regulate development, remains poorly understood. To investigate the role of the maternal microbiome in conditioning the offspring’s stem cells, we manipulated maternal microbiota using Akkermansia muciniphila. Different maternal microbiomes had distinct effects on proliferation and differentiation of neuronal and intestinal stem cells in the offspring, influencing their developmental trajectory, physiology, and long-term health. Transplantation of altered maternal microbiota into germ-free mice transmitted these stem cell phenotypes to the recipients’ offspring. The progeny of germ-free mice selectively colonized with Akkermansia did not display these stem cell traits, emphasizing the importance of microbiome diversity. Metabolically more active maternal microbiomes enriched the levels of circulating short-chain fatty acids (SCFAs) and amino acids, leaving distinct transcriptomic imprints on the mTOR pathway of offsprings’ stem cells. Blocking mTOR signaling during pregnancy eliminated the maternal-microbiome-mediated effects on stem cells. These results suggest a fundamental role of the maternal microbiome in programming offsprings’ stem cells and represent a promising target for interventions.
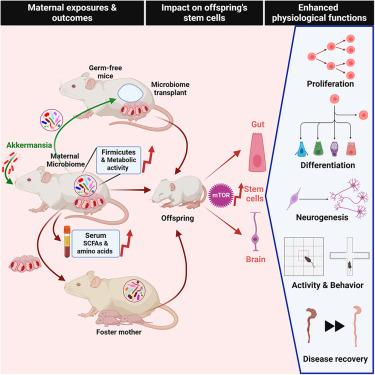
母体肠道微生物群影响后代干细胞功能
母体微生物组影响儿童健康。然而,它对后代干细胞的影响,调控发育,仍然知之甚少。为了研究母体微生物群在调节后代干细胞中的作用,我们使用嗜粘Akkermansia muciniinia操纵母体微生物群。不同的母体微生物组对后代神经元和肠道干细胞的增殖和分化有不同的影响,影响它们的发育轨迹、生理和长期健康。将改变的母体微生物群移植到无菌小鼠体内,将这些干细胞表型传递给受体的后代。选择性定植Akkermansia的无菌小鼠的后代没有表现出这些干细胞特征,强调了微生物组多样性的重要性。代谢更活跃的母体微生物组丰富了循环短链脂肪酸(SCFAs)和氨基酸的水平,在后代干细胞的mTOR通路上留下了明显的转录组印记。在怀孕期间阻断mTOR信号可以消除母体微生物组介导的对干细胞的影响。这些结果表明,母体微生物组在后代干细胞的编程中起着基本作用,并代表了一个有希望的干预目标。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
文献相关原料
公司名称
产品信息
麦克林
KH2PO4
阿拉丁
L-threonine
阿拉丁
L-threonine
阿拉丁
L-threonine
阿拉丁
L-threonine
阿拉丁
L-threonine
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


