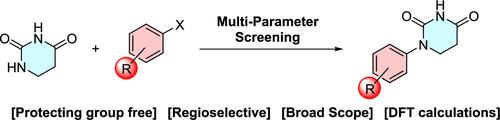
One-Step Regioselective Synthesis of N-1-Substituted Dihydrouracils: A Motif of Growing Popularity in the Targeted Protein Degradation Field
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The increasing popularity of the dihydrouracil motif in cereblon (CRBN) recruiting proteolysis-targeting chimeras (PROTACs) has necessitated the development of a facile, cost-effective, and high-yielding method for its introduction into molecules. To that end, we disclose herein an N-1 selective Pd-catalyzed cross-coupling of dihydrouracil with aryl electrophiles to provide access to medicinally relevant scaffolds in a single step. This approach exhibits excellent functional group tolerance and broad applicability to an abundance of (hetero)aryl halides and phenol derivatives and utilizes readily available catalyst/ligand systems. Thus, our strategy should find broad utility in the arena of PROTAC research, as it obviates the drawbacks of previous methodologies that rely on multistep synthetic routes and protecting group strategies to achieve N-1 selectivity.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


