Multigram Synthesis of 3,3-Spiro-α-prolines
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A series of novel spirocyclic α-proline building blocks with a spiro conjunction in position 3 of the pyrrolidine ring was prepared to employ two convenient and practical synthetic approaches. Both alternative routes utilize simple and readily available starting materials─cyclic ketones and esters─and comprise 6 and 7 steps, respectively. The methodologies feature distinct advantages, using routine organic chemistry transformations, and are suitable for producing multigram amounts of the target prolines. The approach also became valuable for spirocyclic pyroglutamic acids.
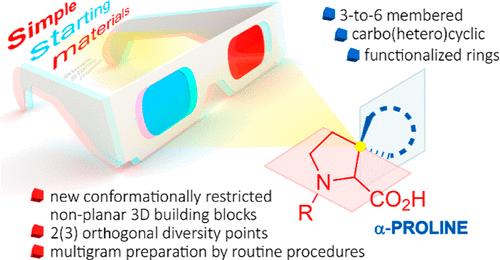
3,3-螺旋-α-脯氨酸的复合合成
采用两种方便实用的合成方法,制备了吡咯烷环3位有螺旋连接的新型螺旋环α-脯氨酸构建块。这两种方法都使用简单且容易获得的起始材料──环酮和酯──分别包括6步和7步。该方法具有明显的优势,使用常规的有机化学转化,并适用于生产多克量的目标脯氨酸。该方法对螺环焦谷氨酸也很有价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


