Refining α-Spodumene with Potassium Sulfate Compared to the Conventional Sulfuric Acid Process
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
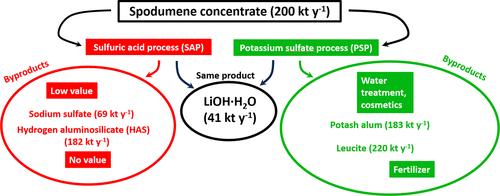
The conventional process of extracting lithium by decrepitating the concentrates of α-spodumene and then baking β-spodumene with concentrated sulfuric acid is the most economic to operate but nonsustainable because of its feedstock, energy, and waste byproduct intensity. Directly extracting lithium from α-spodumene by roasting it with potassium sulfate (K2SO4) followed by leaching it with water offers a more sustainable alternative. We optimized the potassium sulfate process (PSP) at a ratio of K2SO4 to spodumene concentrate of 0.6:1 (w/w), 1050 °C, and 30 min roasting time, achieving a lithium extraction efficiency of 96.3 ± 1.4% (w/w), in comparison to 96.66 ± 0.37% (w/w) for the conventional process, for the same feedstock of spodumene concentrate. While the purification of the leach liquor from PSP is more complex, requiring the addition of aluminum sulfate to recover potassium as potash alum, its byproducts have high economic value. Both processes display a similar energy demand, based on 200 kt y–1 of the spodumene concentrate feed. The use of aluminum sulfate increases the overall cost of PSP by $12.8 million, but sales of potassium alum elevate the revenue by $45.8 million. We reveal that the key advantage of PSP lies in its capability of leveraging the byproducts (leucite and potash alum), while the sulfuric acid process (SAP) may incur disposal cost for its hydrogen aluminosilicate (HAS) byproduct. For PSP to breakeven with SAP, leucite must be converted to a fertilizer and sold at a price of $102.6 t–1 if HAS requires no disposal cost. With the process development focused on the byproduct value, the proposed PSP provides an efficient, more sustainable, and potential near zero-waste alternative to the conventional refining of the lithium chemicals from spodumene.
硫酸法精炼α-锂辉石的比较研究
传统的α-锂辉石精矿先焙烧后用浓硫酸焙烧提取锂的工艺是最经济的,但由于原料、能源和废副产物强度的限制,是不可持续的。用硫酸钾(K2SO4)焙烧后用水浸出α-锂辉石中锂是一种更可持续的方法。对硫酸钾工艺(PSP)进行了优化,在K2SO4与锂辉石精矿的比例为0.6:1 (w/w)、焙烧温度为1050℃、焙烧时间为30 min的条件下,在相同的锂辉石精矿原料条件下,常规工艺的锂提取效率为96.66±0.37% (w/w),而硫酸钾工艺的锂提取效率为96.3±1.4% (w/w)。而PSP浸出液的提纯较为复杂,需要添加硫酸铝回收钾作为钾矾,其副产物具有较高的经济价值。根据锂辉石精矿进料的200kt - 1计算,这两种工艺显示出相似的能源需求。硫酸铝的使用使PSP的总成本增加了1280万美元,但钾明矾的销售使收入增加了4580万美元。我们揭示了PSP的关键优势在于其利用副产品(白色石和钾明矾)的能力,而硫酸法(SAP)可能会产生处理氢铝硅酸盐(HAS)副产品的成本。为了使PSP与SAP实现收支平衡,如果HAS不需要处理成本,则白铁矿必须转化为肥料并以102.6美元的价格出售。随着工艺开发的重点放在副产品价值上,拟议的PSP提供了一种高效、更可持续、潜在的接近零废物的替代方法,可以从锂辉石中提炼锂化学品。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


