Discovery of 6-Fluoro-5-{4-[(5-fluoro-2-methyl-3-oxo-3,4-dihydroquinoxalin-6-yl)methyl]piperazin-1-yl}-N-methylpyridine-2-carboxamide (AZD9574): A CNS-Penetrant, PARP1-Selective Inhibitor
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
PARP inhibitors have attracted considerable interest in drug discovery due to the clinical success of first-generation agents such as olaparib, niraparib, rucaparib, and talazoparib. Their success lies in their ability to trap PARP to DNA; however, first-generation PARP inhibitors were not strictly optimized for trapping nor for selectivity among the PARP enzyme family. Previously we described the discovery of the second-generation PARP inhibitor AZD5305, a selective PARP1-DNA trapper. AZD5305 maintained the antitumor efficacy of first-generation PARP inhibitors while exhibiting lower hematological toxicity. Recently, there has been interest in central nervous system (CNS)-penetrant PARP inhibitors for CNS malignancies and other neurological conditions; however, AZD5305 is not CNS penetrant. Herein we describe the discovery and optimization of a series of CNS-penetrant, PARP1-selective inhibitors and PARP1-DNA trappers, culminating in the discovery of AZD9574, a compound that maintains the PARP1 selectivity of AZD5305 with improved permeability, reduced efflux, and increased CNS penetration.
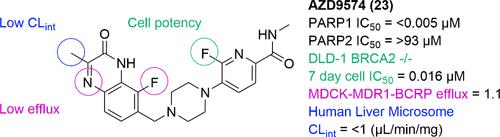
6-氟-5-{4-[(5-氟-2-甲基-3-氧-3,4-二氢喹啉-6-基)甲基]哌嗪-1-基}- n-甲基吡啶-2-羧酰胺(AZD9574):一种cns渗透、parp1选择性抑制剂的发现
由于第一代药物如奥拉帕尼、尼拉帕尼、鲁卡帕尼和塔拉唑帕尼的临床成功,PARP抑制剂在药物发现方面吸引了相当大的兴趣。它们的成功在于它们能够将PARP捕获到DNA上;然而,第一代PARP抑制剂并没有严格优化PARP酶家族的捕获和选择性。之前我们描述了第二代PARP抑制剂AZD5305的发现,这是一种选择性PARP1-DNA诱捕剂。AZD5305维持了第一代PARP抑制剂的抗肿瘤效果,同时具有较低的血液毒性。最近,人们对中枢神经系统(CNS)渗透PARP抑制剂治疗中枢神经系统恶性肿瘤和其他神经系统疾病产生了兴趣;然而,AZD5305不是CNS渗透剂。在本文中,我们描述了一系列CNS渗透、PARP1选择性抑制剂和PARP1- dna捕集剂的发现和优化,最终发现了AZD9574,该化合物通过提高渗透性、减少外排和增加CNS渗透来保持AZD5305的PARP1选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


