Differential HBV RNA and HBcrAg patterns in untreated patients with chronic hepatitis delta
IF 26.8
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background and aim
Serum HBV RNA and HBcrAg levels have been proposed as useful biomarkers in the management of HBV patients, however their role in chronic hepatitis Delta (CHD) is currently unknown.Methods
Consecutive untreated CHD patients were enrolled in a cross-sectional study in three EU centers. Clinical and virological characteristics were collected. Serum HBV RNA and HBcrAg levels were quantified by an automated real-time investigational assay (Cobas® 6800, Roche Diagnostics, Pleasanton, Ca, USA) and by LUMIPULSE® G HBcrAg assay (Fujirebio Europe), respectively. In 18 patients with available liver biopsies, intrahepatic analyses were performed.Results
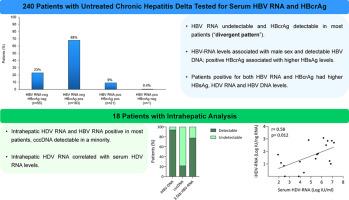
Overall, 240 HDV patients were enrolled: median age 46 years, 62% males, 53% cirrhotics, 57% NUC-treated, median ALT 70 U/L, HBsAg 3.8 log10 IU/mL, 88% HBeAg-negative, median HDV RNA 4.9 log10 IU/mL. HBV RNA tested positive (>10 cp/mL) in only 8% of the patients [median 40 (13-82,000) cp/mL], whereas HBcrAg was ≥3 log10 U/mL in 77% [median 4.2 (3.0-8.0) log10 U/mL]. By combining these biomarkers, 3 categories were identified: 23% double negative (HBV RNA neg/HBcrAg neg), 9% double positive (HBV RNA pos/HBcrAg pos) and 68% HBV RNA negative/HBcrAg positive. HBV RNA levels positively correlated with male sex and detectable HBV DNA, while positive HBcrAg correlated with higher HBsAg levels. Double positive patients were younger, non-European, with elevated ALT and HDV RNA levels and detectable HBV DNA. Intrahepatic HDV RNA and HBV RNA were positive in most samples, while intrahepatic levels of covalently closed circular (ccc)DNA were low.Conclusions
In untreated CHD, most patients had undetectable HBV RNA but quantifiable HBcrAg (“divergent pattern”) in the absence of HBeAg. Additional studies aimed to unravel the molecular mechanisms underlying these findings are warranted.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hepatology
医学-胃肠肝病学
CiteScore
46.10
自引率
4.30%
发文量
2325
审稿时长
30 days
期刊介绍:
The Journal of Hepatology is the official publication of the European Association for the Study of the Liver (EASL). It is dedicated to presenting clinical and basic research in the field of hepatology through original papers, reviews, case reports, and letters to the Editor. The Journal is published in English and may consider supplements that pass an editorial review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


