Highly Porous Scandium(III) Tetrafluoroisophthalate Framework for Adsorptive Separation of Light Alkanes
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
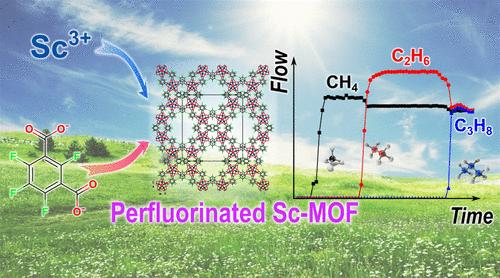
The separation of light alkanes is one of the most important tasks for modern industry due to the widespread use of ethane and propane as chemical feedstocks. Their extraction from natural gas is a challenging task and is now carried out by cryogenic distillation at a limited number of plants around the world. The development of new materials for adsorption separation is therefore important. Among the different types of adsorbents, metal–organic frameworks (MOFs) are one of the most interesting due to their infinite design possibilities. Here we present a highly porous perfluorinated MOF [Sc(OH)(1,3-tFbdc)] (1,1,3-tFbdc2–─1,3-C6F4(COO)22– tetrafluoroisophthalate linker) with a BET surface area greater than 1000 m·g–1 and its ability to separate light alkanes was investigated. The ability of 1 to separate light hydrocarbons at 0 and 25 °C is demonstrated by IAST calculation of selectivity factors as well as by dynamic breakthrough experiments. The role of fluorine substituents within the organic linker of MOF 1 in gas adsorption is revealed by quantum chemical calculations.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


