Study on the Effect and Mechanism of Support and Deposition-Precipitation Method on Ru-Based Catalysts for Ammonia Decomposition
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
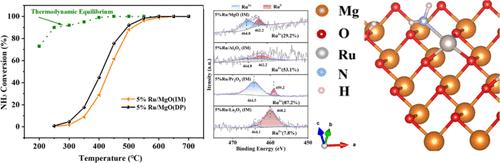
Herein, the effects of support and the deposition-precipitation method on the Ru-based catalysts for NH3 decomposition were studied. The results of the performance test, characterization, and DFT simulation show that the activity order of the catalysts with different supports is 5% Ru/MgO > 5% Ru/Al2O3 > 5% Ru/Pr2O3 > 5% Ru/La2O3. Ru/MgO exhibits the best ammonia decomposition performance (T80 ≈ 480 °C), because its suitable pore structure is conducive to ammonia adsorption, and abundant strong alkaline sites produce a strong metal–support interaction. The ammonia decomposition performance of 5% Ru/MgO (DP) prepared by the deposition-precipitation method is much higher than that of 5% Ru/MgO (IM) prepared by the impregnation method (T80 decreases from 480 to 440 °C). On Ru/MgO (DP), the distribution of Ru particles is more uniform and the particle size is relatively consistent, and the Ru/MgO (DP) has more basic sites and a more reasonable ratio of lattice oxygen to defect oxygen. Calculated by DFT, the energy barrier of the first dehydrogenation of NH3 and the combined desorption of N is 1.31 and 1.51 eV, respectively, and the latter is the rate-determining step of the ammonia decomposition reaction in Ru/MgO.
载体与沉积沉淀法对ru基氨分解催化剂的影响及机理研究
研究了载体和沉积沉淀法对ru基NH3分解催化剂性能的影响。性能测试、表征和DFT模拟结果表明,不同载体催化剂的活性顺序为5% Ru/MgO >;5% Ru/Al2O3 >;5% Ru/Pr2O3 >;俄文/ La2O3 5%。Ru/MgO表现出最佳的氨分解性能(T80≈480℃),因为其合适的孔隙结构有利于氨吸附,并且丰富的强碱性位点产生了强的金属-载体相互作用。沉积沉淀法制备的5% Ru/MgO (DP)的氨分解性能远高于浸渍法制备的5% Ru/MgO (IM) (T80从480℃降低到440℃)。在Ru/MgO (DP)上,Ru颗粒的分布更加均匀,粒径也相对一致,并且Ru/MgO (DP)具有更多的碱性位,晶格氧与缺陷氧的比例更加合理。通过DFT计算,NH3第一次脱氢和N联合脱附的能垒分别为1.31和1.51 eV,后者是Ru/MgO中氨分解反应的速率决定步骤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


