Low-Temperature Phase Separation of CO2 from Syngas Mixtures─Experimental Results
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
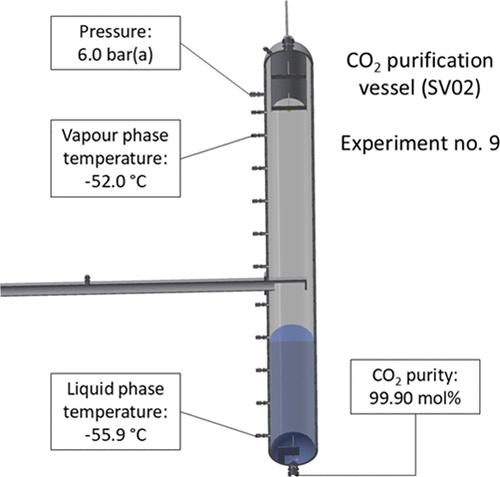
To mitigate the increasing anthropogenic CO2 emissions, hydrogen is pointed to as a potential low-emission alternative fuel for a range of applications. Gray hydrogen from natural gas reforming is the dominant industrial hydrogen source globally. If CO2 capture and storage can be added with minimal efficiency reductions, blue hydrogen can reduce the carbon footprint drastically. A novel technology option for blue hydrogen production, is the use of proton conducting membranes for hydrogen purification combined with low-temperature condensation and phase separation of CO2 from the retentate gas. This work presents results from 15 experiments for low-temperature phase separation and purification of CO2 from five-component mixtures representative for retentate and tail gas compositions. The experiments have been conducted with feed rates between 120 and 307 kg/h and with CO2 feed fractions between 56 and 82 mol %. The main separator pressures and temperatures range between 40 and 70 bar, and −55 and −45 °C, respectively. Final CO2 product purities up to 99.90 mol % have been measured. The purity can be controlled through the pressure level in the flash purification separator and the temperature upstream of the inlet throttling valve. Experiments and corresponding predictions based on GERG-2008 and Peng–Robinson are generally consistent.
合成气混合物中CO2的低温相分离─实验结果
为了减轻日益增加的人为二氧化碳排放,氢被指出是一种潜在的低排放替代燃料,可用于一系列应用。天然气重整制灰氢是全球主要的工业氢源。如果能以最小的效率降低增加二氧化碳的捕获和储存,蓝氢就能大幅减少碳足迹。蓝色氢气生产的一种新技术是使用质子导电膜进行氢气净化,并结合低温冷凝和从保留气体中分离CO2。这项工作介绍了15个低温相分离和净化CO2的实验结果,这些实验来自于代表保留液和尾气成分的五组分混合物。实验条件为进料速率为120 ~ 307 kg/h, CO2进料分数为56 ~ 82 mol %。主分离器的压力和温度范围分别为40 ~ 70bar, - 55 ~ - 45℃。最终的二氧化碳产品纯度达到99.90摩尔%。纯度可以通过闪蒸净化分离器内的压力水平和进口节流阀上游的温度来控制。基于geg -2008和Peng-Robinson的实验和相应预测基本一致。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


