Bridging the Gap between Molecular Simulations and Surface Complexation Modeling for Heterogeneous Surfaces: A Case Study with Uranium and Arsenic Adsorption on Clay Minerals
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
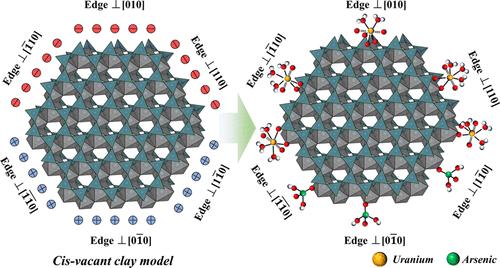
Among all natural submicrosized phases, clay minerals are ubiquitous in soils and sedimentary rocks in nature as well as in engineered environments, and while clay minerals’ adsorption properties have been studied extensively, their unique level of surface reactivity heterogeneities necessitates further investigation at the molecular level to understand and predict the influence of these heterogeneities on their macroscopic properties. In this study, we investigated the surface structures and desorption-free energies of U(VI) species (UO22+) and As(V) species (H2AsO4– and HAsO42–) complexed at different edge surface reactive sites of a cis-vacant montmorillonite layer using first-principles molecular dynamics (FPMD). We show that U(VI) forms bidentate and tridentate complexes on montmorillonite edge surfaces, whereas As(V) monodentate complexes are the most stable. Then, we constrained a state-of-the-art surface complexation model (SCM) with surface acid–base chemistry and surface complexation properties obtained from the molecular-level simulation results. While our results highlighted the complexity of the interfacial chemistry controlling the complexation of inorganic contaminants on clay mineral surfaces, they also evidenced the reliability of an integrated workflow using modern multiscale simulation techniques to address the challenge of predicting heterogeneities in mineral surface reactivity.
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


