Multi-omic profiling highlights factors associated with resistance to immuno-chemotherapy in non-small-cell lung cancer
IF 31.7
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
Abstract
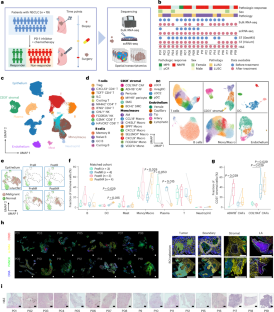
Although immune checkpoint blockade (ICB) therapies have shifted the treatment paradigm for non-small-cell lung cancer (NSCLC), many patients remain resistant. Here we characterize the tumor cell states and spatial cellular compositions of the NSCLC tumor microenvironment (TME) by analyzing single-cell transcriptomes of 232,080 cells and spatially resolved transcriptomes of tumors from 19 patients before and after ICB–chemotherapy. We find that tumor cells and secreted phosphoprotein 1-positive macrophages interact with collagen type XI alpha 1 chain-positive cancer-associated fibroblasts to stimulate the deposition and entanglement of collagen fibers at tumor boundaries, obstructing T cell infiltration and leading to poor prognosis. We also reveal distinct states of tertiary lymphoid structures (TLSs) in the TME. Activated TLSs are associated with improved prognosis, whereas a hypoxic microenvironment appears to suppress TLS development and is associated with poor prognosis. Our study provides novel insights into different cellular and molecular components corresponding to NSCLC ICB–chemotherapeutic responsiveness, which will benefit future individualized immuno-chemotherapy. Samples from 19 patients with non-small-cell lung cancer treated with a combination of chemotherapy and immune checkpoint blockade are profiled with single-cell RNA sequencing and spatial transcriptomics to identify factors associated with treatment resistance.

多组学分析强调与非小细胞肺癌免疫化疗耐药相关的因素
尽管免疫检查点阻断(ICB)疗法已经改变了非小细胞肺癌(NSCLC)的治疗模式,但许多患者仍然具有耐药性。本文通过分析19例患者icb化疗前后232,080个细胞的单细胞转录组和肿瘤的空间分解转录组,表征了肿瘤细胞状态和肿瘤微环境(TME)的空间细胞组成。我们发现肿瘤细胞和分泌的磷酸蛋白1阳性巨噬细胞与XI α 1型胶原链阳性的癌相关成纤维细胞相互作用,刺激胶原纤维在肿瘤边界的沉积和缠结,阻碍T细胞浸润,导致预后不良。我们还揭示了TME中三级淋巴结构(TLSs)的不同状态。激活的TLS与预后改善有关,而缺氧微环境似乎抑制TLS的发育并与预后不良有关。我们的研究对与NSCLC icb化疗反应性相关的不同细胞和分子成分提供了新的见解,这将有利于未来的个体化免疫化疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


