T cell dynamics with neoadjuvant immunotherapy in head and neck cancer
IF 81.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
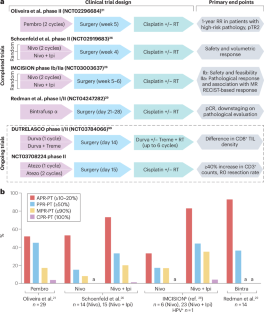
Immune-checkpoint inhibitors (ICIs) are being tested as neoadjuvant therapies in various solid tumours, including in patients with head and neck squamous cell carcinoma (HNSCC), with promising results. Key findings thus far include that this approach is well-tolerated with favourable clinical outcomes including promising pathological response rates in initial studies. Pathological responses are likely to be increased by combining other agents with anti-PD-(L)1 antibodies. Comparisons of baseline biopsy samples with post-treatment surgical specimens have enabled correlative studies utilizing multiomic and immunogenomic methods. Data from these studies suggest that pretreatment intratumoural tissue-resident memory CD8+ T cells are key drivers of tumour regression and give rise to both local and systemic antitumour immune responses. Analyses of systemic responses have defined a PD-1+KLRG1− circulating CD8+ T cell subpopulation that is highly predictive of response, and revealed the interrelationships between intratumoural clones and circulating CD8+ T cells. Lastly, interrogation of T cell populations within lymph nodes is beginning to delineate the immune crosstalk between the primary tumour and tumour-draining lymph nodes and how this relationship might be disrupted with tumour infiltration of the latter. In this Review, we examine data from trials testing neoadjuvant ICIs in patients with HNSCC, focusing on human papillomavirus-unrelated disease, and highlight correlative immunogenomic findings from these trials. Neoadjuvant administration of immune-checkpoint inhibitors (ICIs) has substantially improved the outcomes in patients with various solid tumours, including those with head and neck cancer. However, not all patients derive benefit, indicating a need for biomarkers that enable accurate predictions of a response to these agents. In this Review the authors describe changes in both intratumour and circulating T cells in patients with locally advanced head and neck cancer receiving neoadjuvant ICIs, and consider the role of specific T cell subsets as biomarkers in this setting.

T细胞动力学与新辅助免疫治疗头颈癌
免疫检查点抑制剂(ICIs)作为各种实体肿瘤的新辅助疗法,包括头颈部鳞状细胞癌(HNSCC)患者,正在进行测试,并取得了令人满意的结果。迄今为止的主要发现包括这种方法耐受性良好,临床结果良好,包括初步研究中有希望的病理反应率。其他药物联合抗pd -(L)1抗体可能会增加病理反应。基线活检样本与治疗后手术标本的比较使得利用多组学和免疫基因组学方法进行相关研究成为可能。这些研究的数据表明,预处理肿瘤内组织驻留记忆CD8+ T细胞是肿瘤消退的关键驱动因素,并引起局部和全身抗肿瘤免疫反应。系统反应分析定义了PD-1+KLRG1 -循环CD8+ T细胞亚群,该亚群可高度预测反应,并揭示了肿瘤内克隆与循环CD8+ T细胞之间的相互关系。最后,对淋巴结内T细胞群的调查开始描绘原发性肿瘤和肿瘤引流淋巴结之间的免疫串扰,以及这种关系如何随着肿瘤浸润而被破坏。在这篇综述中,我们研究了在HNSCC患者中检测新辅助ICIs的试验数据,重点关注与人乳头瘤病毒无关的疾病,并强调了这些试验中相关的免疫基因组学发现。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


